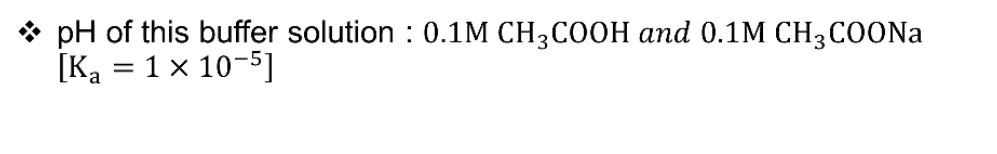
Question: pH of this buffer solution : 0.1M CH3COOH and 0.1M CH3COONa [Ka = 1 x 10-5] 10 L 0.1M CH3COOH solution is mixed with 4
![[Ka = 1 x 10-5] 10 L 0.1M CH3COOH solution is mixed](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f91d2ecf73d_96666f91d2e7dbad.jpg)
pH of this buffer solution : 0.1M CH3COOH and 0.1M CH3COONa [Ka = 1 x 10-5] 10 L 0.1M CH3COOH solution is mixed with 4 L 0.1M NaOH. Find out the pH - [pKa = 4.5] =
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


