Question: Phosphorus and bromine react to form phosphorus tribromide, like this: P4(g)+6Br2(g)4PBr3(g) Also, a chemist finds that at a certain temperature the equillbrium mixture of phosphorus,
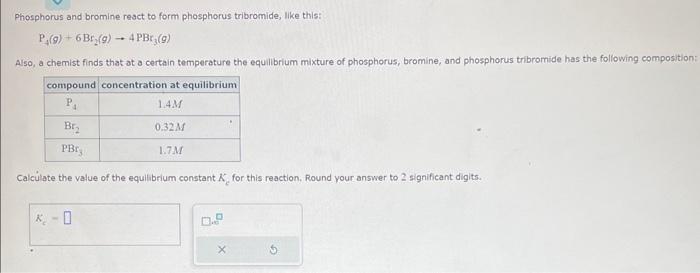
Phosphorus and bromine react to form phosphorus tribromide, like this: P4(g)+6Br2(g)4PBr3(g) Also, a chemist finds that at a certain temperature the equillbrium mixture of phosphorus, bromine, and phosphorus tribromide has the foliowing composition: Calculate the value of the equilibrium constant Kc for this reaction. Round your answer to 2 significant digits
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


