Question: Physical Chemistry Please use a program (Excel, MATLAB etc) for the graph do NOT hand draw it please Background revision from CHMI 2516/2526E: The Henry
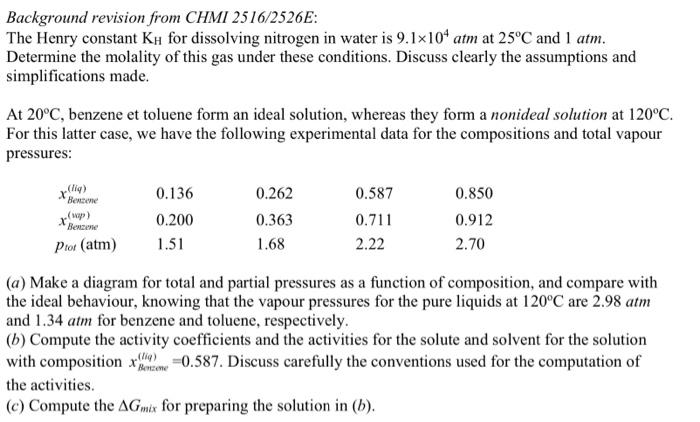
Background revision from CHMI 2516/2526E: The Henry constant KH for dissolving nitrogen in water is 9.1104atm at 25C and 1atm. Determine the molality of this gas under these conditions. Discuss clearly the assumptions and simplifications made. At 20C, benzene et toluene form an ideal solution, whereas they form a nonideal solution at 120C. For this latter case, we have the following experimental data for the compositions and total vapour pressures: (a) Make a diagram for total and partial pressures as a function of composition, and compare with the ideal behaviour, knowing that the vapour pressures for the pure liquids at 120C are 2.98atm and 1.34atm for benzene and toluene, respectively. (b) Compute the activity coefficients and the activities for the solute and solvent for the solution with composition xBmaxan(tiq)=0.587. Discuss carefully the conventions used for the computation of the activities. (c) Compute the Gmix for preparing the solution in (b)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


