Question: Physical chemistry question. Please answer clearly. A) Let us define a reduced speed VR=(2kTm)1/2V where v is speed. Trasform the Maxwell probality function for speed
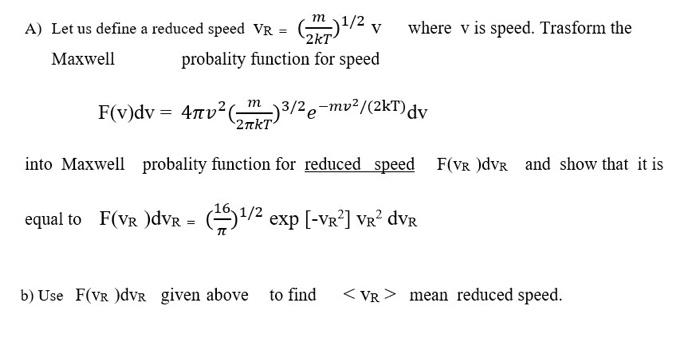
A) Let us define a reduced speed VR=(2kTm)1/2V where v is speed. Trasform the Maxwell probality function for speed F(v)dv=4v2(2kTm)3/2emv2/(2kT)dv into Maxwell probality function for reduced speed F(vR)dvR and show that it is equal to F(vR)dvR=(16)1/2exp[vR2]vR2dvR b) Use F(vR)dvR given above to find vR mean reduced speed. A) Let us define a reduced speed VR=(2kTm)1/2V where v is speed. Trasform the Maxwell probality function for speed F(v)dv=4v2(2kTm)3/2emv2/(2kT)dv into Maxwell probality function for reduced speed F(vR)dvR and show that it is equal to F(vR)dvR=(16)1/2exp[vR2]vR2dvR b) Use F(vR)dvR given above to find vR mean reduced speed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


