Question: Platinum: Platinum crystallizes in a face-centered cubic structure with an edge length of a0=3.9231A. Two Interpenetrating Simple Cubic Lattices FCC Crystal Type BCC Simple Cubic

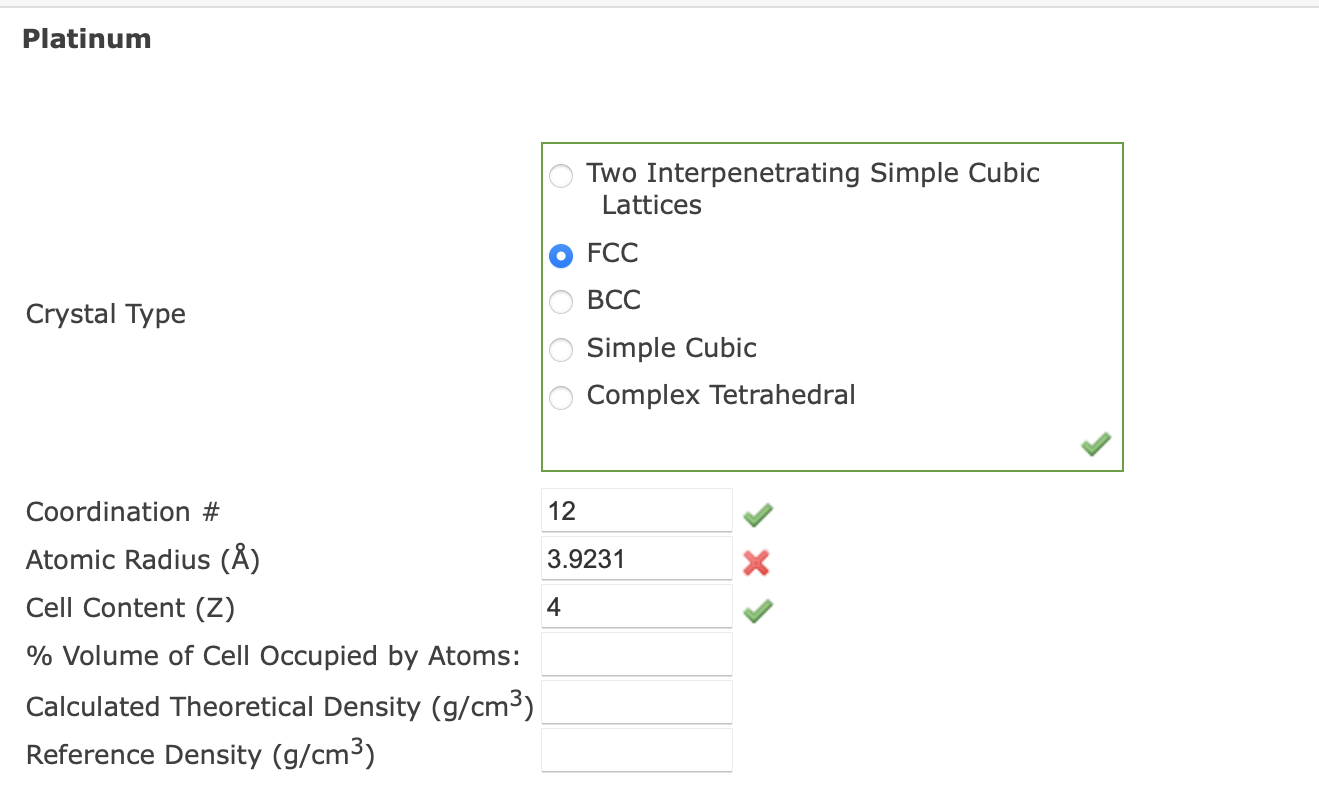
Platinum: Platinum crystallizes in a face-centered cubic structure with an edge length of a0=3.9231A. Two Interpenetrating Simple Cubic Lattices FCC Crystal Type BCC Simple Cubic Complex Tetrahedral
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


