Question: Pleas solve (c, d, e) with the given Data then on (Excell) I need screen shots from(( Excell)) for the solution. Answer ALL of the
Pleas solve (c, d, e) with the given Data then on (Excell) I need screen shots from(( Excell)) for the solution.

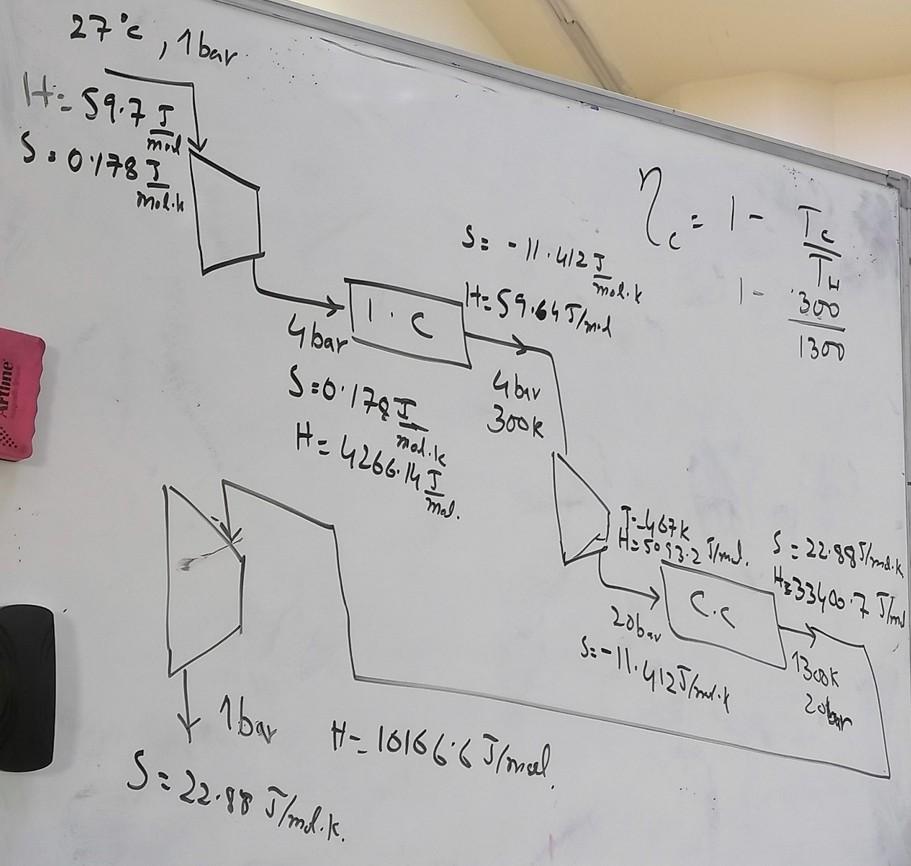
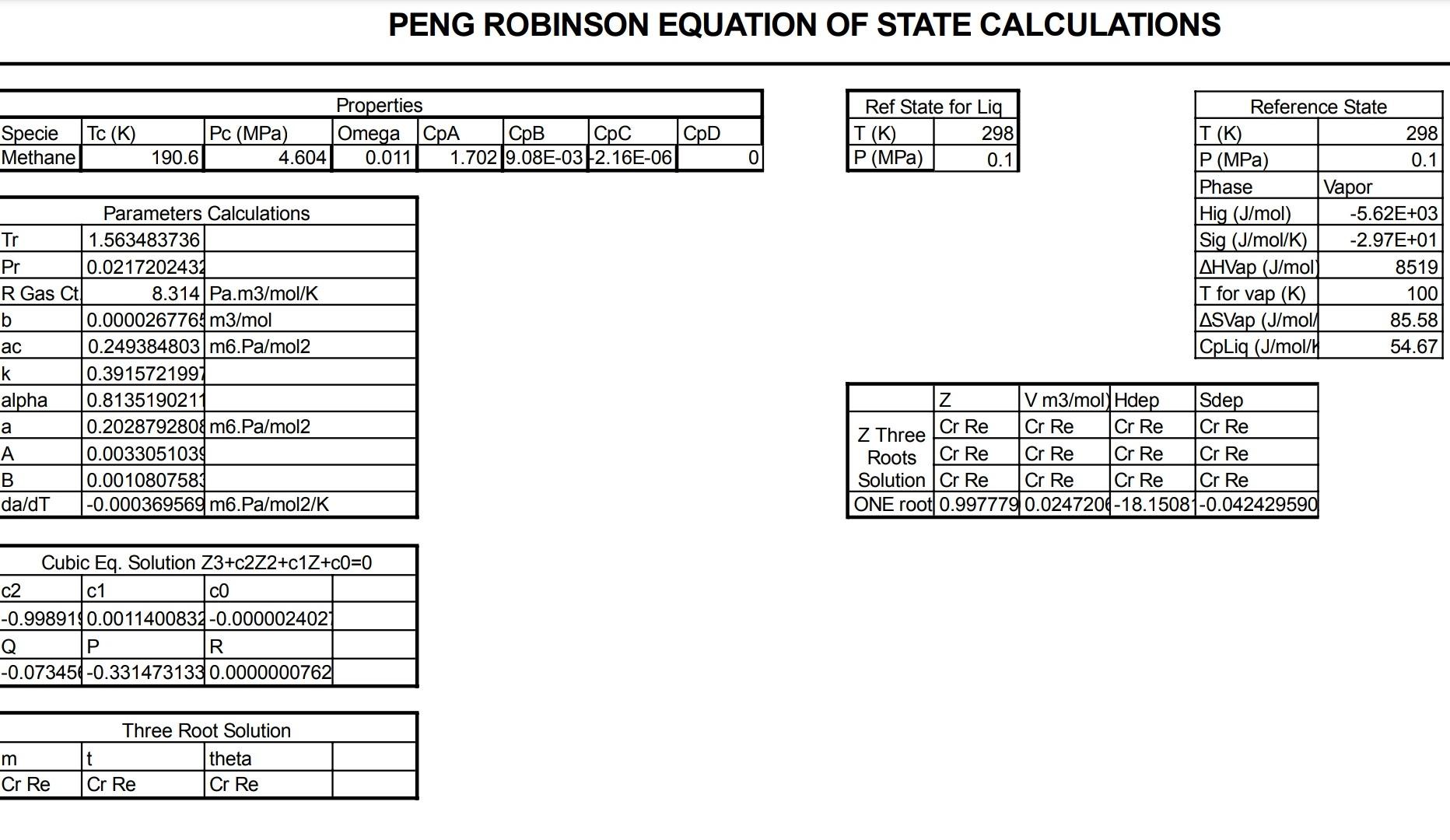
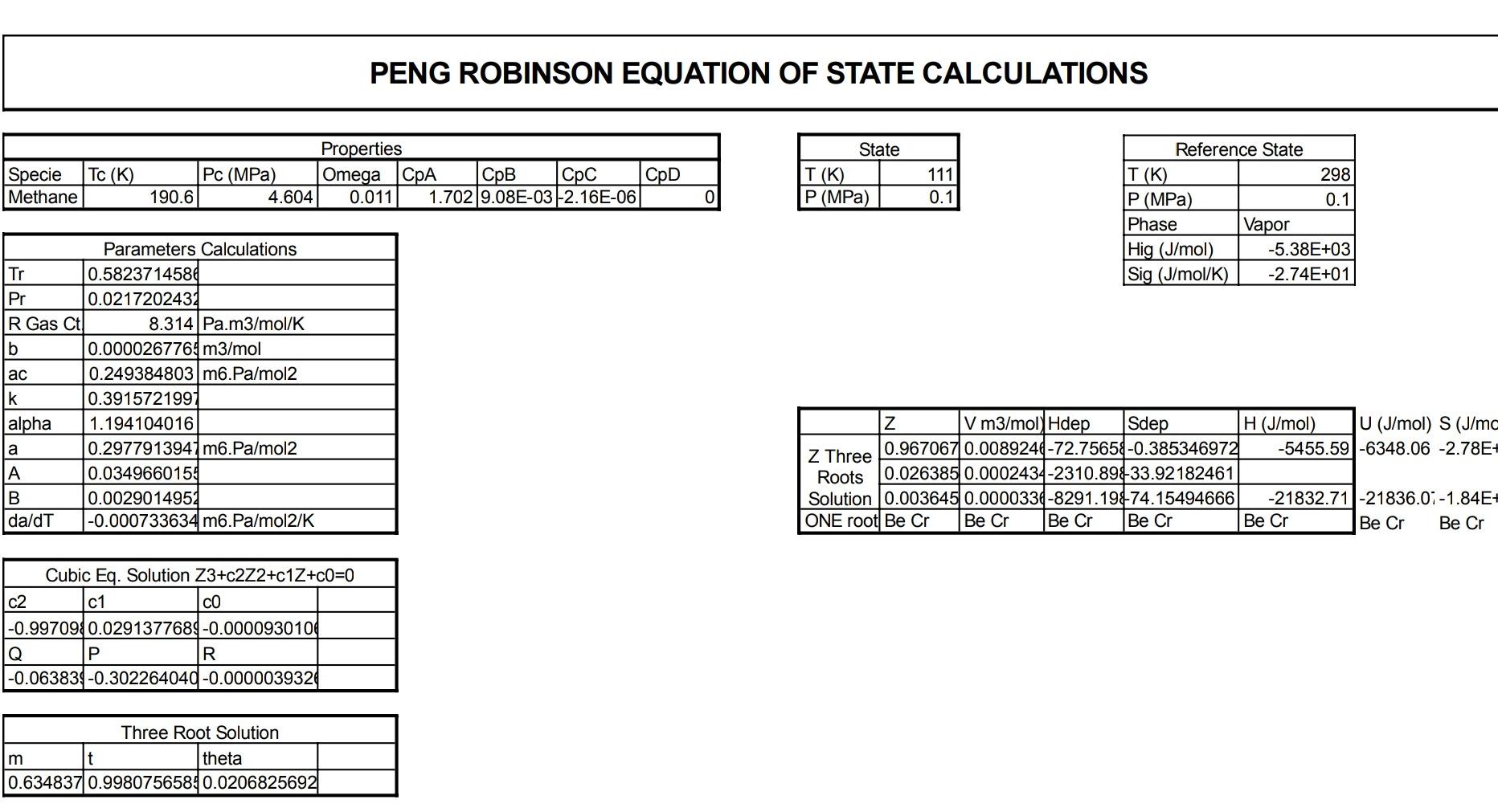
Answer ALL of the following questions Q 01 Suppose a gas turbine is working on pure nitrogen. Nitrogen at 27C and 1 bar is compressed by two stage compressor. Final outlet pressure is recorded as 20 bar. First stage outlet pressure is 4 bar. Temperature of the nitrogen is raised to 1300 K in the heater (external combustion chamber) before expansion in the turbine to 1 bar. (Thermodynamic data is provided, use interpolation where required) c) The cycle generates 50 MW of energy, what is the flow rate of nitrogen required in m/s at 1 bar and 300K? d) Redo part 'c' if the efficiency of the compressor and turbine are 70 %. e) What should be the temperature of nitrogen before entering the turbine to achieve breakeven point of efficiency (efficiency = 0) of practical/irreversible cycle? = 27c, 1 bar H: 59.7I 3.0178I modi Molih REI-T S: - 11.412 I molik H: 59.645/and 300 4 bar! 1300 Alline jo 1795 hodie :0 I molle H = 4266.14 I Gbar 300k mal. 2 0 53.2 md. C. 5:22997 4+33400 75lm Lobar S: -11.4125 bondik J1Book zobor 1bay H-101666 Ilmal S=22.88 J/ml.k. PENG ROBINSON EQUATION OF STATE CALCULATIONS TC (K) Specie Methane Properties Pc (MPa) Omega CPA CpB | pc CPD 190.6 4.604 0.011 1.7029.08E-032.16E-06 Ref State for Liq T(K) 298 P (MPa) 0.1 Reference State T(K) 298 P (MPa) 0.1 Phase Vapor Hig (J/mol) -5.62E+03 Sig (J/mol/K) -2.97E+01 AHVap (J/mol 8519 T for vap (K) 100 ASVap (J/mol 85.58 CpLiq (J/mol/l 54.67 Parameters Calculations Tr 1.563483736 Pr 0.0217202431 R Gas Ct 8.314 Pa.m3/mol/K b 0.0000267764 m3/mol ac 0.249384803 m6.Pa/mol2 k 0.3915721997 alpha 0.8135190211 a 0.2028792808m6.Pa/mol2 A 0.0033051039 B 0.0010807583 da/dT -0.000369569 m6.Pa/mol2/K Z V m3/ mol) Hdep Sdep Cr Re Cr Re Cr Re Cr Re Z Three Roots Cr Re Cr Re Cr Re Cr Re Solution Cr Re Cr Re Cr Re Cr Re ONE root 0.997779 0.0247204-18.1508 -0.042429590 Cubic Eq. Solution Z3+c2Z2+c1Z+00=0 c2 c1 CO -0.9989190.0011400834-0.0000024021 Q P R -0.073454-0.331473133 0.0000000762 m Cr Re Three Root Solution t theta Cr Re Cr Re PENG ROBINSON EQUATION OF STATE CALCULATIONS Specie Tc (K) Methane Properties Pc (MPa) Omega CPA CpB pc 4.604 0.011 1.702 9.08E-03 -2.16E-06 CpD State T(K) P (MPa) 111 0.1 190.6 0 Reference State T(K) 298 P (MPa) 0.1 Phase Vapor Hig (J/mol) -5.38E+03 Sig (J/mol/K) -2.74E+01 Parameters Calculations Tr 0.582371458 Pr 0.0217202432 R Gas Ct! 8.314 Pa.m3/mol/K b 0.0000267764 m3/mol ac 0.249384803 m6.Pa/mol2 k 0.3915721997 alpha 1.194104016 0.297791394/ m6.Pa/mol2 0.0349660159 B 0.0029014954 da/dT -0.000733634 mb.Pa/mol2/K Z V m3/mol) Hdep Sdep H (J/mol) U (J/mol S (J/mc Z Three 0.967067 0.0089244-72.75658-0.385346972 -5455.59-6348.06 -2.78E+ Roots 0.026385 0.0002434-2310.8933.92182461 Solution 0.003645 0.0000334-8291.19874.15494666 -21832.71-21836.07-1.84E- ONE root Be Cr Be Cr Be Cr Be Cr Be Cr Be Cr Be Cr Cubic Eq. Solution Z3+c2Z2+c1Z+00=0 c2 c1 CO |-0.99709 0.0291377684-0.0000930104 Q R -0.063839-0.302264040 -0.000003932 Three Root Solution m t theta 0.634837 0.99807565840.0206825692
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


