Question: pleas solve step by step Numerical The reversible reaction N2O42NO2 is carried out at 2atm. The equilibrium constant at 340K is 0.1mol/L and can be
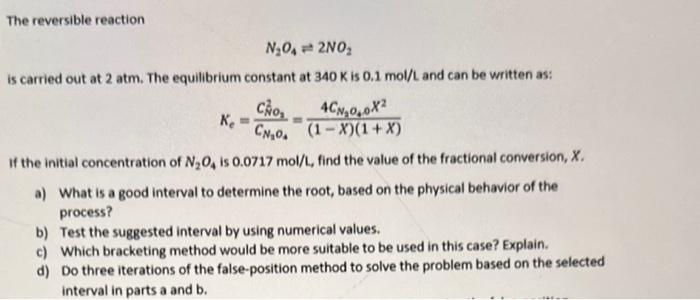
The reversible reaction N2O42NO2 is carried out at 2atm. The equilibrium constant at 340K is 0.1mol/L and can be written as: Ke=CN1O4CN02=(1X)(1+X)4CN2O40X2 If the initial concentration of N2O4 is 0.0717mol/L, find the value of the fractional conversion, X. a) What is a good interval to determine the root, based on the physical behavior of the process? b) Test the suggested interval by using numerical values. c) Which bracketing method would be more suitable to be used in this case? Explain. d) Do three iterations of the false-position method to solve the problem based on the selected interval in parts a and b. The reversible reaction N2O42NO2 is carried out at 2atm. The equilibrium constant at 340K is 0.1mol/L and can be written as: Ke=CN1O4CN02=(1X)(1+X)4CN2O40X2 If the initial concentration of N2O4 is 0.0717mol/L, find the value of the fractional conversion, X. a) What is a good interval to determine the root, based on the physical behavior of the process? b) Test the suggested interval by using numerical values. c) Which bracketing method would be more suitable to be used in this case? Explain. d) Do three iterations of the false-position method to solve the problem based on the selected interval in parts a and b
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


