Question: Please advise: Ihave absolutely no idea how to format the equations to even begin getting the answers 3. Complete Table 2 with your experimental data
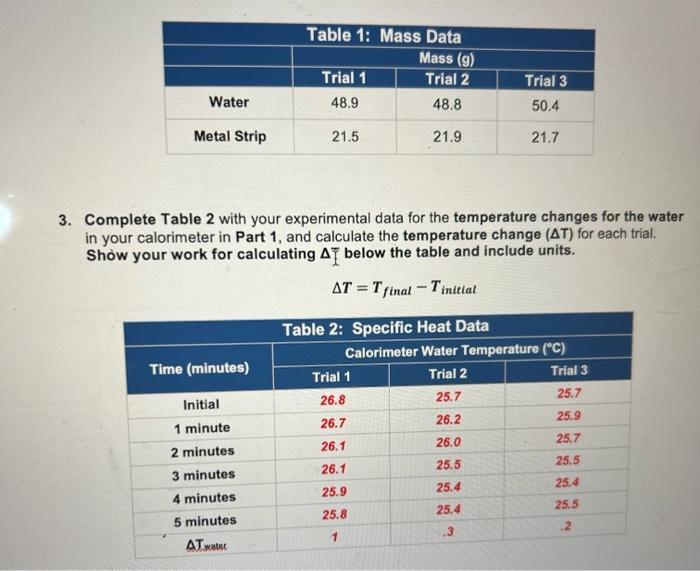

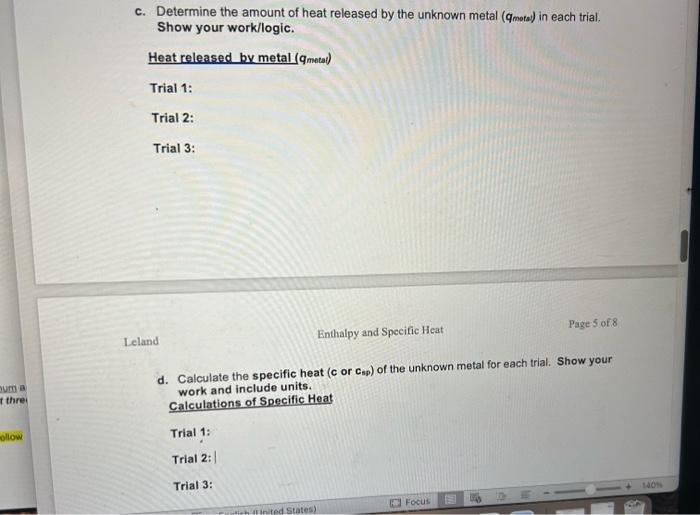
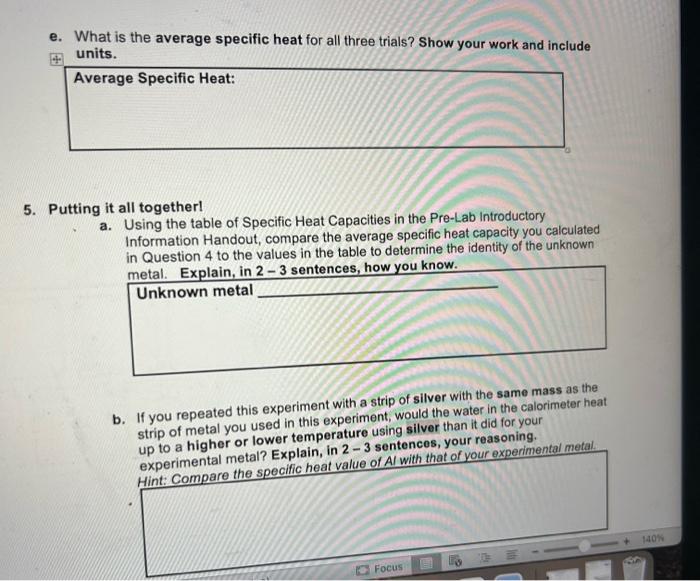
3. Complete Table 2 with your experimental data for the temperature changes for the water in your calorimeter in Part 1 , and calculate the temperature change (T) for each trial. Show your work for calculating below the table and include units. T=TfinalTinitial a. Calculate Tmetalforeachofthethreetrials.Showyourworkandincludeunits: Hint: Table 2 shows the T for the water. For the T of your metal, consider the initial temperature of the metal (Part 1, Step 10 in Procedure) as well as the final temperature once the calorimeter contents reached thermal equilibrium. Calculations of ATmetal66.0C Trial 1: 25.866.0=40.2 Trial 2: 25.4-66.0 =40.6 Trial 3: 25.5-66.0 =40.5 b. Calculate the amount of heat absorbed by the water ( q water) in each trial. Show your work and include units. Calculations of heat absorbed by water ( q water) Trial 1: Trial 2: Trial 3: T=.2 m=50.4 c. Determine the amount of heat released by the unknown metal ( q mots) in each trial. Show your work/logic. Heat released by metal ( qmotal) Trial 1: Trial 2: Trial 3: Leland Enthalpy and Specific Heat Page 5 of 8 d. Calculate the specific heat (c or csp ) of the unknown metal for each trial. Show your work and include units. Calculations of Specific Heat Trial 1: Trial 2: Trial 3: e. What is the average specific heat for all three trials? Show your work and include units. Average Specific Heat: Putting it all togetherl a. Using the table of Specific Heat Capacities in the Pre-Lab Introductory Information Handout, compare the average specific heat capacity you calculated in Question 4 to the values in the table to determine the identity of the unknown metal. Explain, in 23 sentences, how you know. Unknown metal b. If you repeated this experiment with a strip of silver with the same mass as the strip of metal you used in this experiment, would the water in the calorimeter heat up to a higher or lower temperature using silver than it did for your
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


