Question: Please also provide instructions how how to create the plot in Task 2 Project Description: The elementary gas phase reaction A3B is carried out in
Please also provide instructions how how to create the plot in Task 2
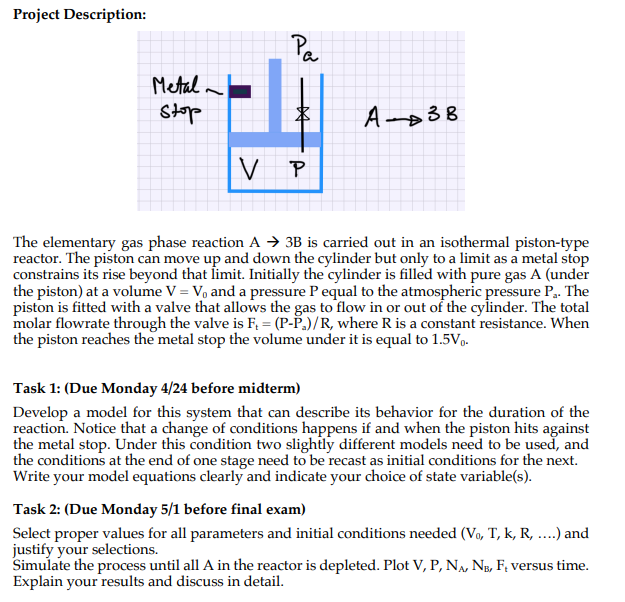
Project Description: The elementary gas phase reaction A3B is carried out in an isothermal piston-type reactor. The piston can move up and down the cylinder but only to a limit as a metal stop constrains its rise beyond that limit. Initially the cylinder is filled with pure gas A (under the piston) at a volume V=V0 and a pressure P equal to the atmospheric pressure Pa. The piston is fitted with a valve that allows the gas to flow in or out of the cylinder. The total molar flowrate through the valve is Ft=(PPa)/R, where R is a constant resistance. When the piston reaches the metal stop the volume under it is equal to 1.5V0. Task 1: (Due Monday 4/24 before midterm) Develop a model for this system that can describe its behavior for the duration of the reaction. Notice that a change of conditions happens if and when the piston hits against the metal stop. Under this condition two slightly different models need to be used, and the conditions at the end of one stage need to be recast as initial conditions for the next. Write your model equations clearly and indicate your choice of state variable(s). Task 2: (Due Monday 5/1 before final exam) Select proper values for all parameters and initial conditions needed (V0,T,k,R,) and justify your selections. Simulate the process until all A in the reactor is depleted. Plot V,P,NA,NB,Ft versus time. Explain your results and discuss in detail
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


