Question: PLEASE AND PLEASE READ THE QUESTION CAREFULLY AND SOLVE THE QUESTION IN GREEN BOX. IT IS DIFFERENT QUESTION AND A NEW QUESTION. PLEASE AND PLEASE
PLEASE AND PLEASE READ THE QUESTION CAREFULLY AND SOLVE THE QUESTION IN GREEN BOX. IT IS DIFFERENT QUESTION AND A NEW QUESTION.
PLEASE AND PLEASE READ THE QUESTION CAREFULLY AND SOLVE THE QUESTION IN GREEN BOX. IT IS DIFFERENT QUESTION AND A NEW QUESTION.
PLEASE AND PLEASE READ THE QUESTION CAREFULLY AND SOLVE THE QUESTION IN GREEN BOX. IT IS DIFFERENT QUESTION AND A NEW QUESTION.
PLEASE DO STEP BY STEP SOLUTION
PLEASE DO STEP BY STEP SOLUTION
PLEASE DO STEP BY STEP SOLUTION
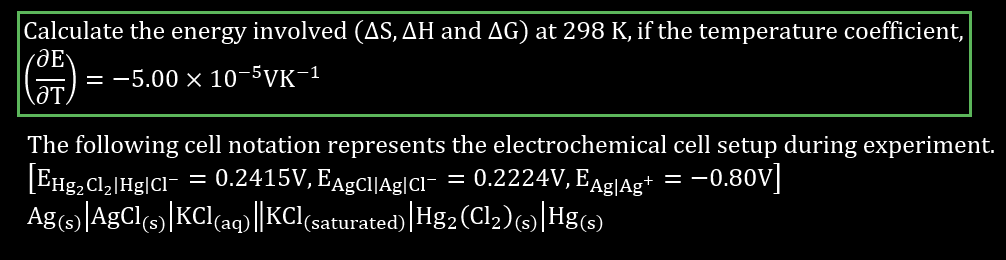
Calculate the energy involved (AS, AH and AG) at 298 K, if the temperature coefficient, = -5.00 x 10-57K-1 The following cell notation represents the electrochemical cell setup during experiment. (EHg2Cl2|Hg/C = 0.2415V, Eagcl/Ag|Cl- = 0.2224V, Eag|Ag+ = -0.80v] Ag(s)|AgCl(s)]KCl(aq)||KCl (saturated)|Hg2 (Cl2)(s) |Hg(s) = =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


