Question: please answee 7-12 7. Which substance would you expect to experience dipole-dipole intermolecular forces? A) thy B) CCl4 c) NF3 D) CS2 E) none of
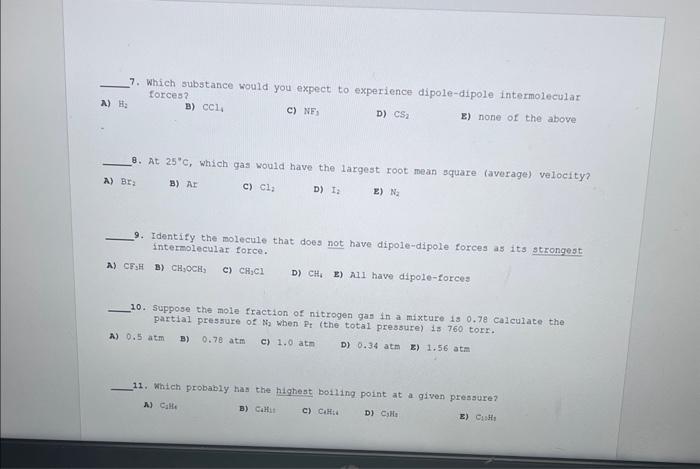

7. Which substance would you expect to experience dipole-dipole intermolecular forces? A) thy B) CCl4 c) NF3 D) CS2 E) none of the above 8. At 25C, which gas would have the largest root mean square (average) velocity? A) Br2 B) NI C) Cl2 D) I2 E) Na2 9. Identify the molecule that does not have dipole-dipole forces as its strongest interelecular force. A) CF3H B) CH2CCH3 c) CH1Cl D) CH6 E) Ali have dipole-forces 10. Suppose the mole fraction of nitrogen gas in a mixture 150.78 calculate the partial pressure of N2 when Pt (the total pressure) 1$760 torr. A) 0.5atm B) 0.78atm c) 1.0atm D) 0.34atm z) 1.56atm 11. Which probably has the highest boiling point at a given pressure? ) Calfe B) CaHit c) Culs:i D) CiH4 a) C1:H1 12. What is the order of increasing density of the following gases at the same temperature and pressure? N2,NH3,N2O6, and Ar A) N2O4
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


