Question: please answer 3,4,5 and 6 Observation Sheet: Effect of Concentration on Rate of Reaction Approximate Molarity of stock crystal violet (CV) solution: 3.00 10Smol/L Wavelength
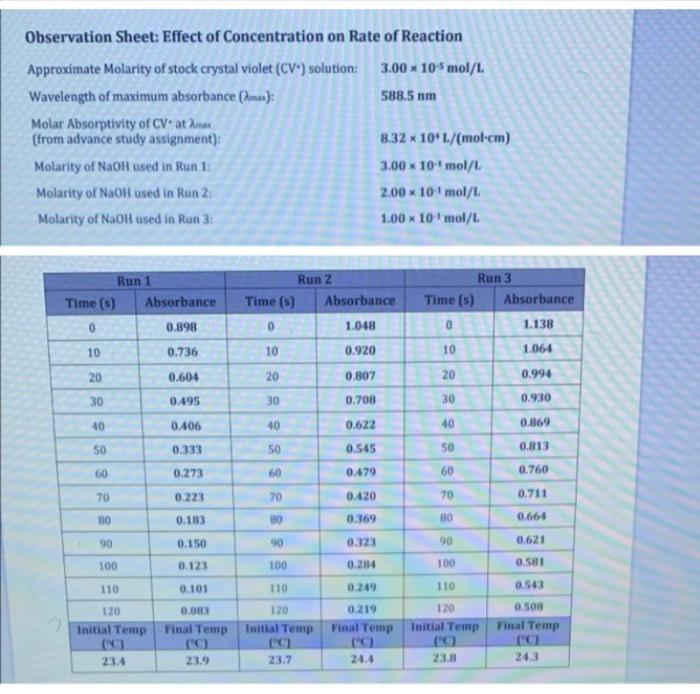
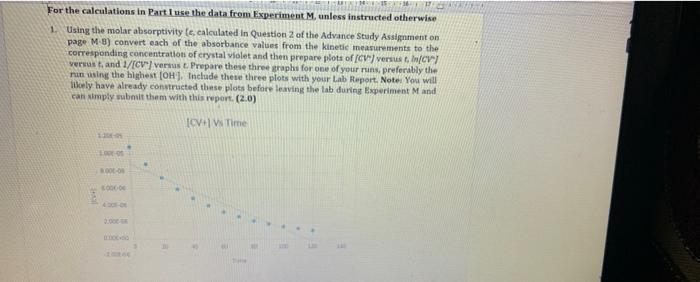

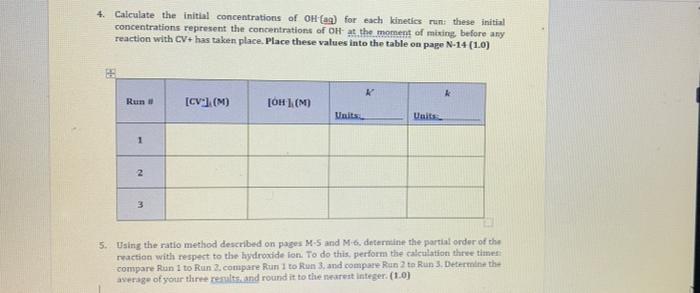

Observation Sheet: Effect of Concentration on Rate of Reaction Approximate Molarity of stock crystal violet (CV) solution: 3.00 10Smol/L Wavelength of maximum absorbance (mas); 588.5 nm Molar Absorptivity of CV atom (from advance study assignment): 8.32 * 109 L/(mol-cm) Molarity of NaOH used in Run 1: 3.00 x 10 mol/L Molarity of NaOH used in Run 2: 2.00 x 10 mol/L Molarity of NaOH used in Run 3: 1.00 x 10 mol/L X Run 1 Time (s) Absorbance 0 0.898 Run 2 Time(s) Absorbance 0 1.048 10 0.920 Run 3 Time (5) Absorbance 0 1.138 10 0.736 10 1.064 20 0.604 20 0.807 20 0.994 30 0.495 30 0.708 30 0.930 40 0.406 40 0.622 40 0.869 50 0.333 0.545 50 0.813 50 60 60 0.479 60 0,760 70 0.273 0.223 0.183 70 0.420 70 0.711 80 80 0.369 HO 0.664 90 0.150 90 0.323 90 0.621 100 0.123 100 0.284 100 0.51 110 110 0.249 110 0.543 0.101 0.083 Final Temp 120 Initial Temp (C) 23.4 120 Initial Temp 120 Taitial Temp 0.500 Final Temp 0.219 Final Temp (C) 24.4 23.9 23.7 23.8 24.3 PG For the calculations in Part I use the data from Experiment M. unless instructed otherwise 1. Using the molar absorptivity le, calculated in Question 2 of the Advance Study Assignment on page MB) convert each of the absorbance values from the kinetic measurements to the corresponding concentration of crystal Violet and then prepare plots of (CVversus In/OV versust, and 1/(CV"verst. Prepare these three straphs for one of your runs, preferably the man using the highest (OH). Include these three plots with your Lab Report. Note: You will Icely have already constructed these plots before leaving the tab during Experiment M and can simply submit them with this report. (2.0) ICVW Time - SOLO 2. Based on your plats in Question what is the order of the action with viet Explain how you determined this (10) And It is the first onder because the graph of NCVVT and the integrated law, the linear all you the order of the plain how you can determine the product for from you prepared in 01 above. Pot the appropriate graph for Run and and write all three values to the table at the top of N-14 e sure to indicate and to your values in an appropriate trumber of candig (10) 4. Calculate the initial concentrations of OH(aq) for each kinetics run these initial concentrations represent the concentrations of OH at the moment of mixing before any reaction with CV has taken place. Place these values into the table on page N-14(1.0) Run [CV] (M) [OH :( Units Units 1 2 3 3 5. Using the ratio method described on pages Mand M. determine the partial order of the reaction with respect to the hydroxide ion. To do this perform the calculation three times compare Run I to Run 2.compare Run 1 to Run 3. and compare Run 2 to Run 3. Determine the average of your three results and round it to the nearest Integer (1.0) 6. Calculate the value of k for each in and add these values to the table at the top of this page and indicate the units for k Show your calculations for Run 1. Determine the average overall rate constant k (1.0)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


