Question: Please answer #6 and #7 If any info is missing that is needed, leave a comment and I will update that. 6. In Part B.
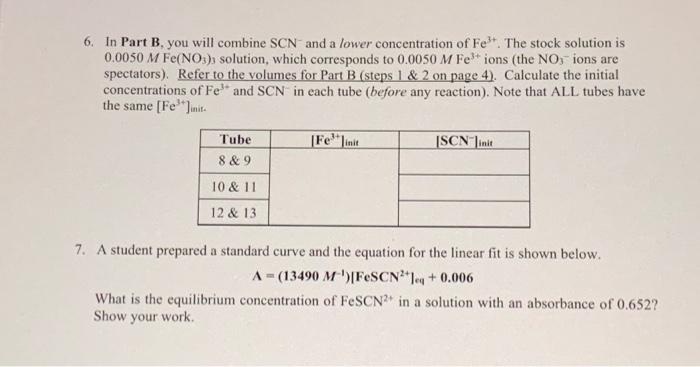
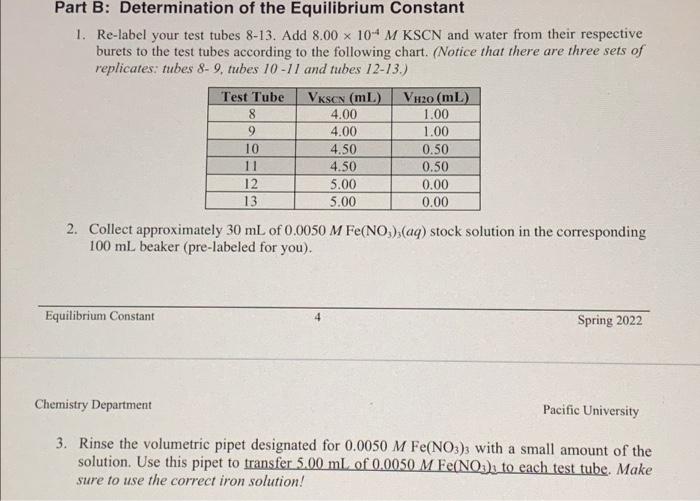
6. In Part B. you will combine SCN and a lower concentration of Fet. The stock solution is 0.0050 M Fe(NO3), solution, which corresponds to 0.0050 M Festions (the NO, ions are spectators). Refer to the volumes for Part B (steps 1 & 2 on page 4). Calculate the initial concentrations of Fe and SCN in each tube (before any reaction). Note that ALL tubes have the same [Fellini Felimit [SCN init Tube 8 & 9 10 & 11 12 & 13 7. A student prepared a standard curve and the equation for the linear fit is shown below. A = (13490 M 'FeSCN1,4 +0.006 What is the equilibrium concentration of FeSCN2 in a solution with an absorbance of 0.652? Show your work Part B: Determination of the Equilibrium Constant 1. Re-label your test tubes 8-13. Add 8.00 x 10-4 M KSCN and water from their respective burets to the test tubes according to the following chart. (Notice that there are three sets of replicates: tubes 8-9, tubes 10-11 and tubes 12-13.) Test Tube 8 9 10 11 12 13 VKSON (ml) 4.00 4.00 4.50 4.50 5.00 5.00 VH20 (mL) 1.00 1.00 0.50 0.50 0.00 0.00 2. Collect approximately 30 mL of 0.0050 M Fe(NOx),(aq) stock solution in the corresponding 100 ml beaker (pre-labeled for you). Equilibrium Constant 4 Spring 2022 Chemistry Department Pacific University 3. Rinse the volumetric pipet designated for 0.0050 M Fe(NO3)3 with a small amount of the solution. Use this pipet to transfer 500 ml of 0.0050 M Fe(NO3), to each test tube. Make sure to use the correct iron solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


