Question: Please answer 8-10 THERE IS NO DATA MISSING! 6. Consider the reaction: 2SO2(g)2SO3(g) for which H=200.kJ and S=186.5J/K at 25C. Assuming that H and S
Please answer 8-10
THERE IS NO DATA MISSING!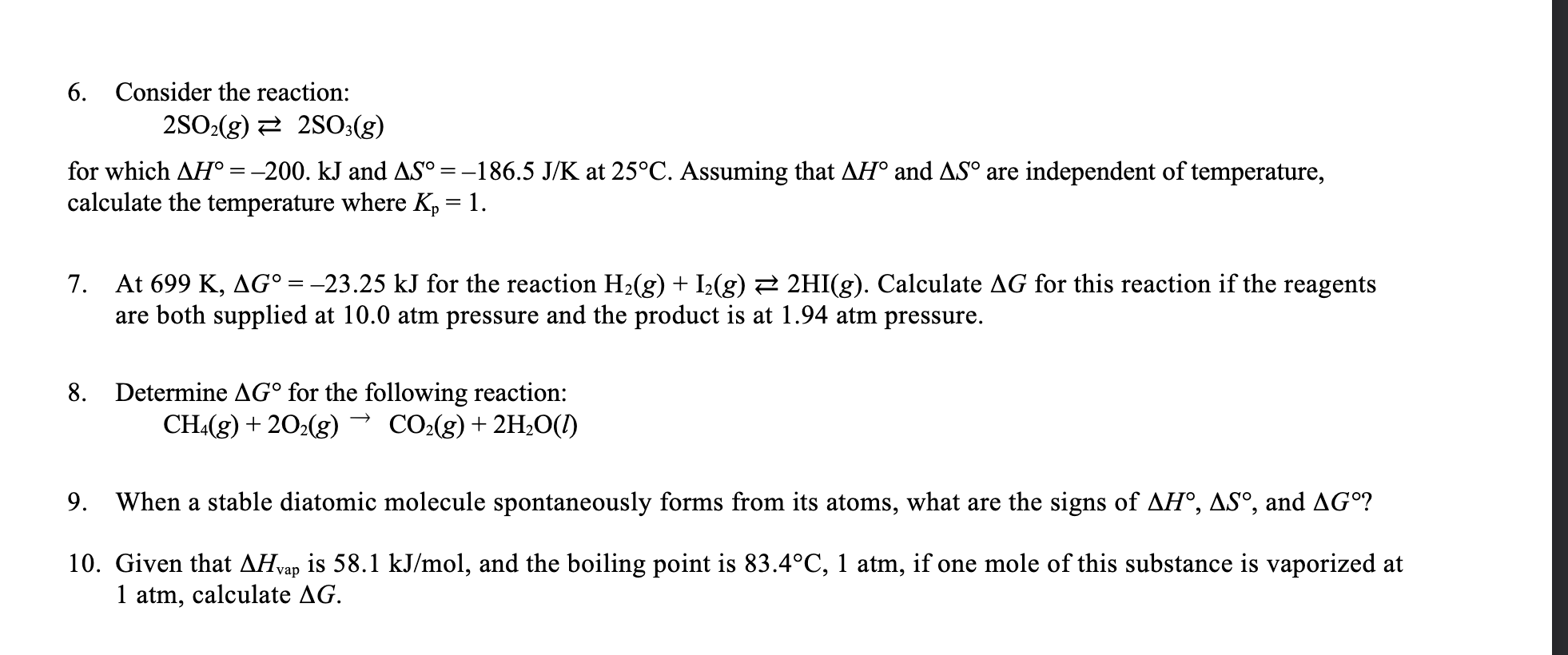
6. Consider the reaction: 2SO2(g)2SO3(g) for which H=200.kJ and S=186.5J/K at 25C. Assuming that H and S are independent of temperature, calculate the temperature where Kp=1. 7. At 699K,G=23.25kJ for the reaction H2(g)+I2(g)2HI(g). Calculate G for this reaction if the reagents are both supplied at 10.0atm pressure and the product is at 1.94 atm pressure. 8. Determine G for the following reaction: CH4(g)+2O2(g)CO2(g)+2H2O(l) 9. When a stable diatomic molecule spontaneously forms from its atoms, what are the signs of H,S, and G ? 10. Given that Hvap is 58.1kJ/mol, and the boiling point is 83.4C,1atm, if one mole of this substance is vaporized at 1 atm, calculate G
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


