Question: please answer all 5 question i really need tge answers thank you:) 14. Fluorine gas exerts a pressure of 900 torr. When the pressure is




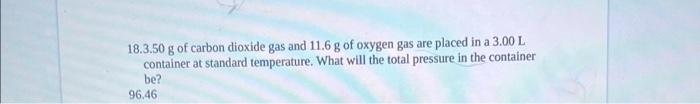
14. Fluorine gas exerts a pressure of 900 torr. When the pressure is changed to 1.50 atm, its volume becomes 250mL. What was the original volume? V1=211 15. A sample of argon gas is cooled and its volume went from 380mL to 250mL. If its final temperature was 55C, what was its original temperature? T1=340 16. A container with a movable piston contains 0.89 liters of gas at 100.5C. If the temperature of the gas rises to 211.3C, what will the new volume be? V2=1.15 17. What volume does 18.9 grams of carbon dioxide gas occupy at 57.5C and 4.35 atm ? 18.3.50 g of carbon dioxide gas and 11.6g of oxygen gas are placed in a 3.00L container at standard temperature. What will the total pressure in the container be? 96.46
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


