Question: please answer all Beer's Law Lab Prelab--Please submit as separate assignment in the Lab Module; This page is posted in Beer's Law Prelab 1. Look
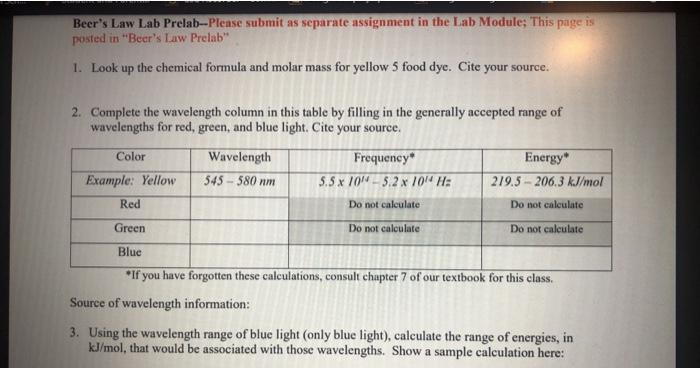
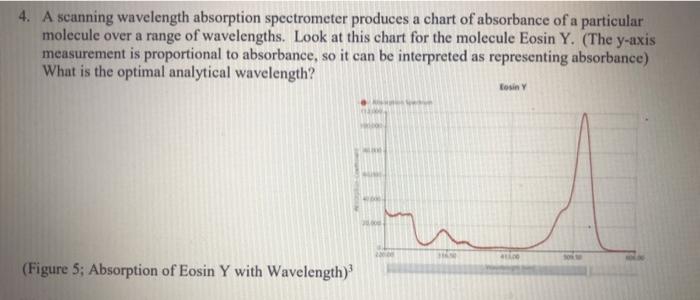
Beer's Law Lab Prelab--Please submit as separate assignment in the Lab Module; This page is posted in "Beer's Law Prelab" 1. Look up the chemical formula and molar mass for yellow 5 food dye. Cite your source. 2. Complete the wavelength column in this table by filling in the generally accepted range of wavelengths for red, green, and blue light. Cite your source. Color Wavelength Frequency Energy" Example: Yellow 545 - 580 nm 5.5 x ION-3,2 x 10W H: 219.5 - 206.3 kJ/mol Red Do not calculate Do not calculate Green Do not calculate Do not calculate Blue *If you have forgotten these calculations, consult chapter 7 of our textbook for this class. Source of wavelength information: 3. Using the wavelength range of blue light (only blue light), calculate the range of energies, in kJ/mol, that would be associated with those wavelengths. Show a sample calculation here: 4. A scanning wavelength absorption spectrometer produces a chart of absorbance of a particular molecule over a range of wavelengths. Look at this chart for the molecule Eosin Y. (The y-axis measurement is proportional to absorbance, so it can be interpreted as representing absorbance) What is the optimal analytical wavelength? Losiny (Figure 5; Absorption of Eosin Y with Wavelength)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


