Question: Please answer all :) Chromium (Cr) is element 24 on the periodic table. Calculate the amount (mol) Cr in 1.83gCr. molCr Ruthenium (Ru) is element
Please answer all :)

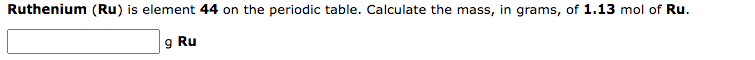

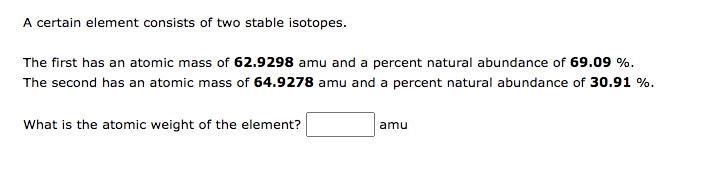
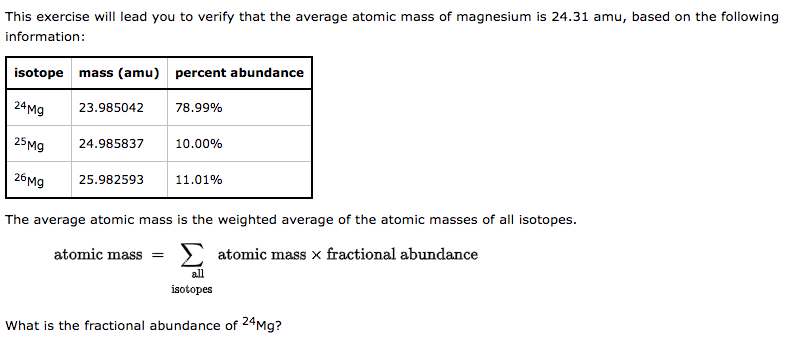
Chromium (Cr) is element 24 on the periodic table. Calculate the amount (mol) Cr in 1.83gCr. molCr Ruthenium (Ru) is element 44 on the periodic table. Calculate the mass, in grams, of 1.13mol of Ru. g Ru of Sm. mol Sm A certain element consists of two stable isotopes. The first has an atomic mass of 62.9298 amu and a percent natural abundance of 69.09%. The second has an atomic mass of 64.9278 amu and a percent natural abundance of 30.91%. What is the atomic weight of the element? amu This exercise will lead you to verify that the average atomic mass of magnesium is 24.31 amu, based on the following information: The average atomic mass is the weighted average of the atomic masses of all isotopes. atomicmass=allisotopesatomicmassfractionalabundance What is the fractional abundance of 24Mg
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


