Question: please answer all for thumps up Using the bond dissociation energies given at the beginning of the exam, estimate the enthalpy change for reaction: CO2(g)C(g)+O2(g).O=C=0i=a
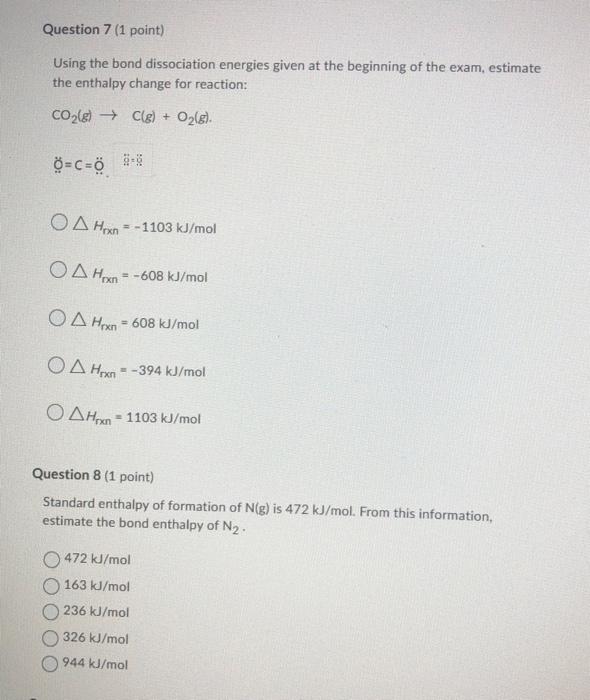
Using the bond dissociation energies given at the beginning of the exam, estimate the enthalpy change for reaction: CO2(g)C(g)+O2(g).O=C=0i=a Hrxn=1103kJ/molHrxn=608kJ/molHrxn=608kJ/molHrxn=394kJ/molHrxn=1103kJ/mol Question 8 (1 point) Standard enthalpy of formation of N(g) is 472kJ/mol. From this information, estimate the bond enthalpy of N2. 472kJ/mol 163kJ/mol 236kJ/mol 326kJ/mol 944kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


