Question: Please answer all multiple choice questions just with answer no explanation needed Answer Box 1. 2. 3. 5. 6. 7. 1. Which of the following
Please answer all multiple choice questions just with answer no explanation needed
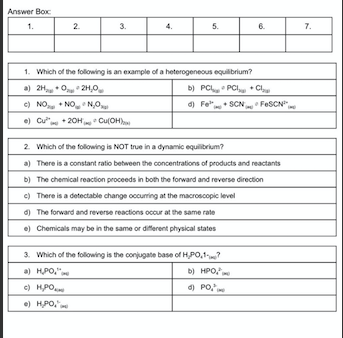
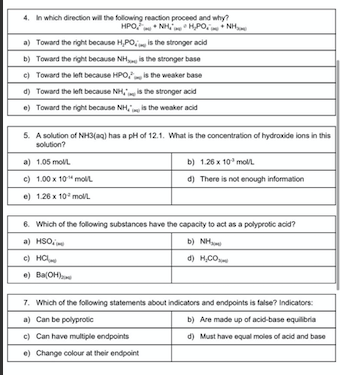
Answer Box 1. 2. 3. 5. 6. 7. 1. Which of the following is an example of a heterogeneous equilibrium? a) 2.024,0 b) PCL.PCC c) NO.NO, NO d) FSCN FeSON +20H Cu(OH) 2. Which of the following is NOT true in a dynamic equilibrium? a) There is a constant ratio between the concentrations of products and reactants b) The chemical reaction proceeds in both the forward and reverse direction c) There is a detectable change occurring at the macroscopic level The forward and reverse reactions occur at the same rate .) Chemicals may be in the same or different physical states 3. Which of the following is the conjugate base of HP0.17 a) HPO. b) HPO, c) HP d) PO, .) HPO. 4. In which direction will the following reaction proceed and why? HPO, NHHPO.N a) Toward the right because H,PO is the stronger aold b) Toward the right because the stronger base c) Toward the left because HPO is the weaker base d) Toward the left because is the stronger acid e) Toward the right because NH, is the weaker adid 5. A solution of NH3(aq) has a pH of 12.1. What is the concentration of hydroxide ions in this solution? a) 1.05 moll b) 126 x 10 moll c) 100 x 10"moll d) There is not enough information e) 126 x 10 mol/l 8. Which of the following substances have the capacity to act as a polyprolic acid? a) HSO b) NH THI c) Hng d) H.COM ) Ba(OH) 7. Which of the following statements about indicators and endpoints is false? Indicators: a) Can be polyprotic b) Are made up of acid base equilibria c) Can have multiple endpoints d) Must have equal moles of acid and base e) Change colour at their endpoint
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


