Question: Please answer ALL. No explanation needed. Thumbs up will be given . Thanks! For the reaction, Cl2(1)+2Na(s)2NaCl(s), the rate of the forward reaction could be
Please answer ALL. No explanation needed. Thumbs up will be given . Thanks!



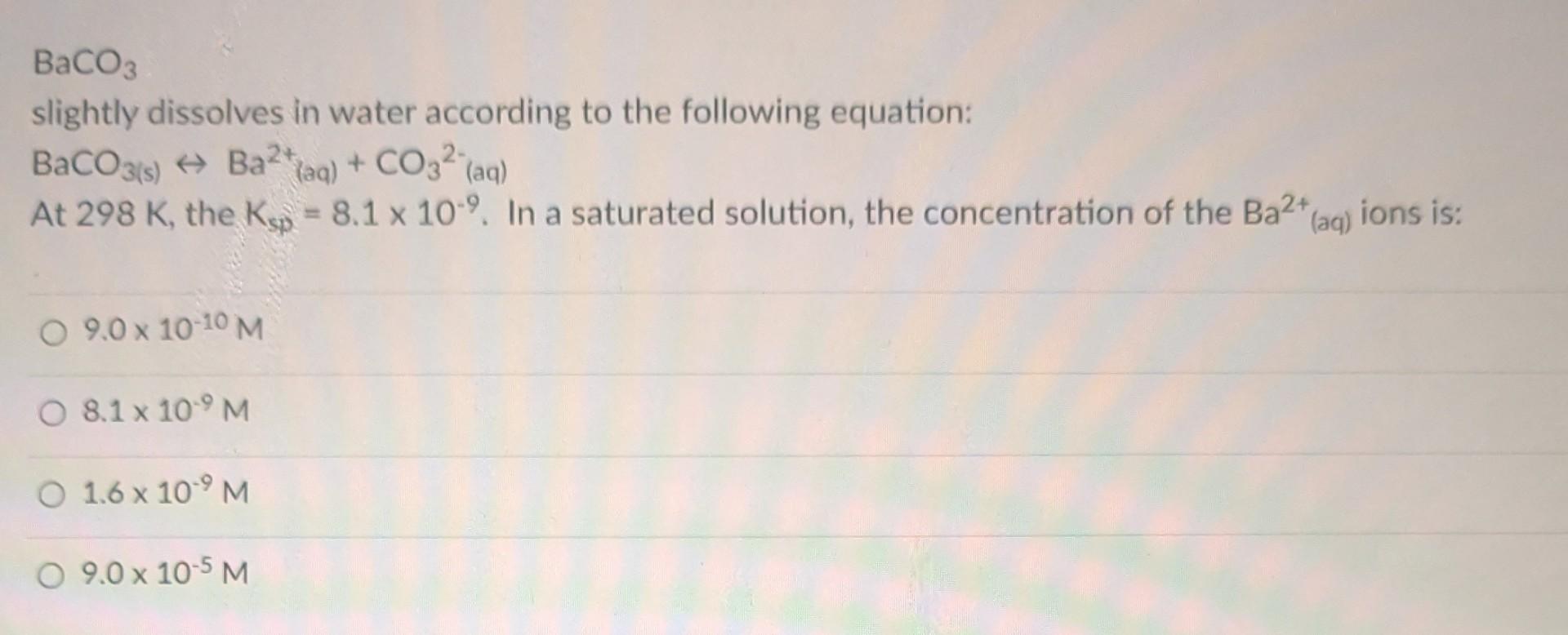
For the reaction, Cl2(1)+2Na(s)2NaCl(s), the rate of the forward reaction could be increased most by: adding Na cooling the system removing NaCl using gaseous Cl2 In which of the following reactions does HNO3 act as a base? Cu+4HNO3Cu2++2NO3+2H2O+2NO2 HNO3H++NO3 HNO3+H2OH3O++NO3 HNO3+H2SO4H2NO3++HSO4 For the equilibrium reaction, HSO4+HPO42SO42+H2PO4 a conjugated Brnsted-Lowry acid-base pair is: HPO42 (acid)and H2PO4(base) HPO42 (base) and H2PO4(acid) HSO4(acid) and HPO42 (base) HSO4- (base) and SO4(acid) slightly dissolves in water according to the following equation: BaCO3(s)Ba2+(aq)+CO32(aq) At 298K, the Ksp=8.1109. In a saturated solution, the concentration of the Ba2+(aq) ions is: 9.01010M8.1109M1.6109M9.0105M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


