Question: please answer all Part III: Short Answer Questions: Show all calculations, chemistry concepts behind all answers and steps for full credit 26. A species has


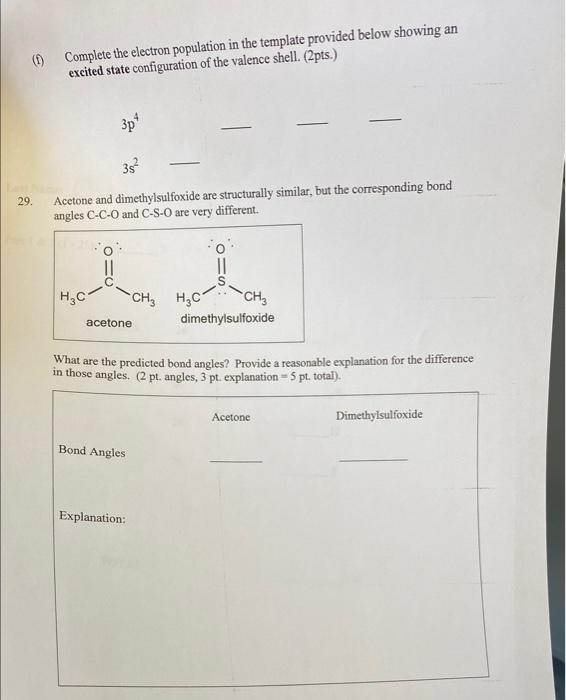
Part III: Short Answer Questions: Show all calculations, chemistry concepts behind all answers and steps for full credit 26. A species has the structure drawn below. Assuming that -bonds are formed using hybrid orbitals and that lone pair electrons reside also in hybrid orbitals, answer the following questions based on the Lewis structure below: A What is the hybridization of the unspecified atom A? (3 pt.) B What is the name for the 3D-shape of the polyatomic species? (3 pt.) C Calculate the formal charge on each of the three oxygen atoms ( 2pt.) D If the overall charge on the polyatomic species is -1, calculate the formal charge on A? (2 pt.) E Based on your answer in 26D, determine the group in the periodic table to which the element A belongs and explain why by showing the calculation of the group number ( 2 pt.) 27. In the molecule drawn below, three protons are marked as 1,2 and 3 on N,C and O respectively. Which one of these marked protons is the most acidic ( 2 pts) and provide the chemical reason why ( 4pts) ? 28. The following is the electron configuration of the valence shell of an element on the Periodic Table. 3s21111 Answer the following questions based on this electron configuration: (a) How many covalent bonds can this atom form in its compounds? (2 pt.) (b) What type of ion will this atom form and what will be its charge? (4 pts.) (c) To what period does this element belong? ( 1 pt.) 12 pts. (d) To what group does this element belong? (1 pt) (e) What are the name and symbol for this element? (2 pts.) (I) Complete the electron population in the template provided below showing an excited state configuration of the valence shell. ( 2pts.) 3p43s2 29. Acetone and dimethylsulfoxide are structurally similar, but the corresponding bond angles CCO and CSO are very different. What are the predicted bond angles? Provide a reasonable explanation for the difference in those angles. ( 2pt. angles, 3pt. explanation =5pt. total)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


