Question: please answer all parts and show work 1. Consider a function Vm=PRT, where R is a constant and P,Vm, and T are independent variables. This
please answer all parts and show work
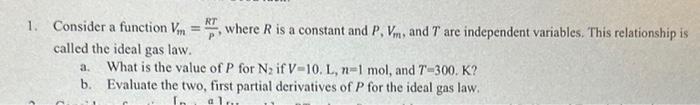
1. Consider a function Vm=PRT, where R is a constant and P,Vm, and T are independent variables. This relationship is called the ideal gas law. a. What is the value of P for N2 if V=10.L,n=1mol, and T=300. K ? b. Evaluate the two, first partial derivatives of P for the ideal gas law
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


