Question: Please answer all parts to question 2, table 1 is also given 2) Answer the following questions as they relate to the redox reaction below:

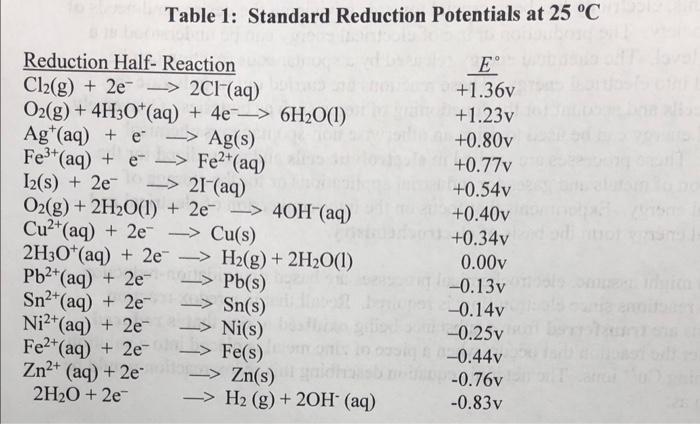
2) Answer the following questions as they relate to the redox reaction below: a) Write the oxidation half-reaction for the redox reaction shown above b) Write the reduction half-reaction for the redox reaction shown above c) Use the half-reactions written above to determine the coefficients needed to balance the redox equation. Place the appropriate coefficients in the blanks. d) Use Table 1 in the introduction, along with the half-reactions, to determine E cell for the overall reaction (Assume the reaction is spontaneous as written). e) Use the Nernst equation to calculate the cell potential if [Fe2+]=0.20M, [Fe3+]=0.30M,[Cl]=0.10M and P(Cl2)=1.0atm at 25C. Table 1: Standard Reduction Potentials at 25C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


