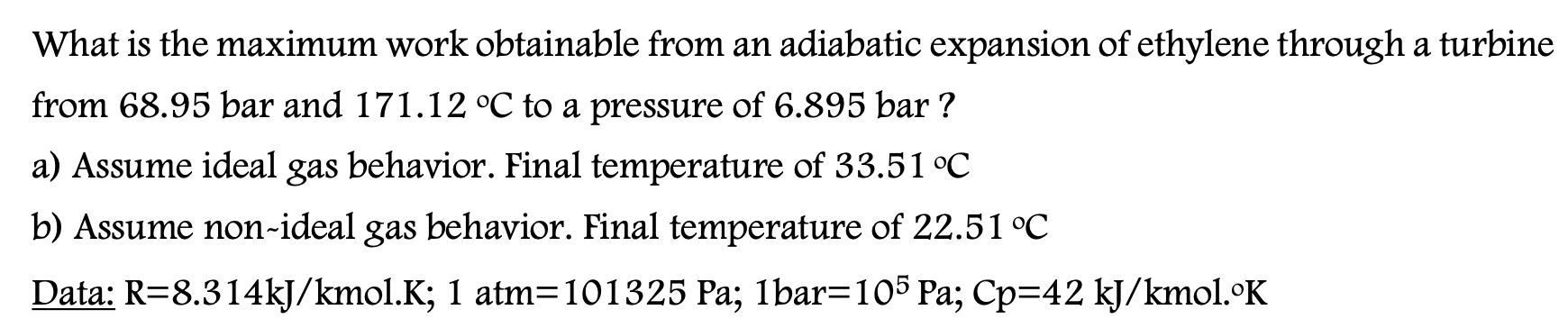
Question: Please answer all parts. What is the maximum work obtainable from an adiabatic expansion of ethylene through a turbine from 68.95 bar and 171.12 C
 Please answer all parts.
Please answer all parts.
What is the maximum work obtainable from an adiabatic expansion of ethylene through a turbine from 68.95 bar and 171.12 C to a pressure of 6.895 bar ? a) Assume ideal gas behavior. Final temperature of 33.51 C b) Assume non-ideal gas behavior. Final temperature of 22.51 C Data: R=8.314kJ/kmol.K; 1 atm=101325 Pa; lbar=105 Pa; Cp=42 kJ/kmol.OK
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


