Question: please answer all problems Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it
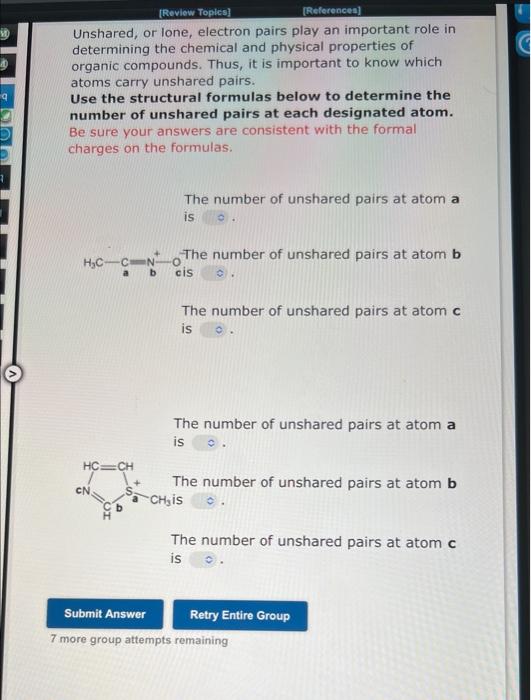

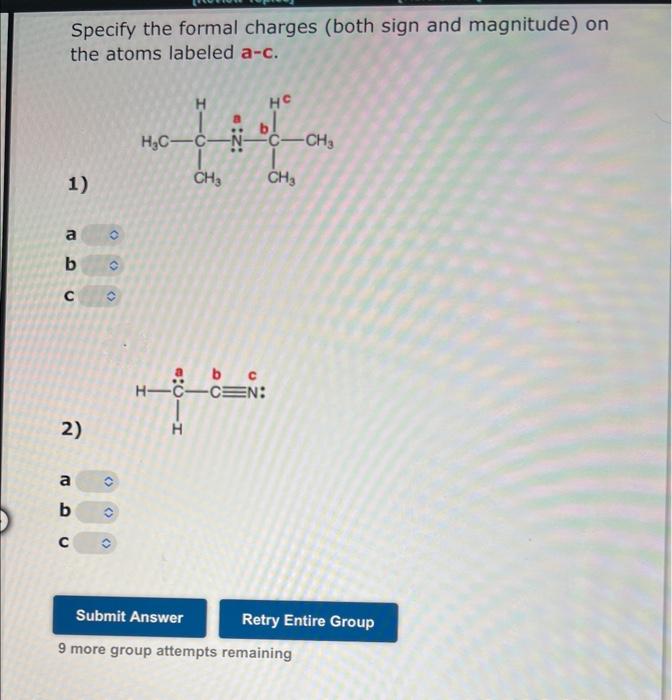
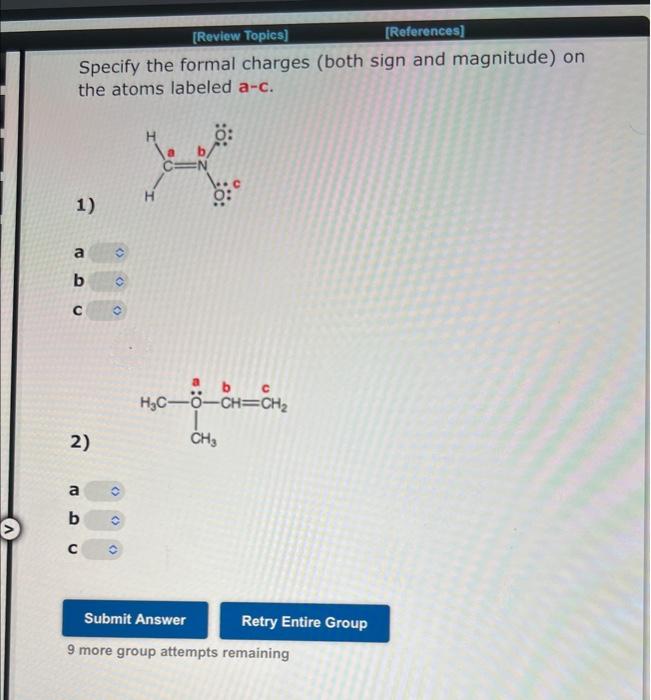
Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. The number of unshared pairs at atom a is H3CCaN+OThe number of unshared pairs at atom b cis The number of unshared pairs at atom c is The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c is 7 more group attempts remaining [Review Topics] [Relerences] Unshared, or lone, electron pairs play an important role in determining the chemical and physical properties of organic compounds. Thus, it is important to know which atoms carry unshared pairs. Use the structural formulas below to determine the number of unshared pairs at each designated atom. Be sure your answers are consistent with the formal charges on the formulas. The number of unshared pairs at atom a is The number of unshared pairs at atom b is The number of unshared pairs at atom c is The number of unshared pairs at atom a is b - The number of unshared pairs at atom b is The number of unshared pairs at atom c is 7 more group attempts remaining Specify the formal charges (both sign and magnitude) on the atoms labeled a-c. 1) a b c 2) a b c 9 more group attempts remaining Specify the formal charges (both sign and magnitude) on the atoms labeled a-c. 1) a b c 2) a b c 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


