Question: Please answer all questions. Don't copy paste any answer from chegg or I will vote down + If the answer is incomplete, I'll vote down.
Please answer all questions. Don't copy paste any answer from chegg or I will vote down + If the answer is incomplete, I'll vote down.
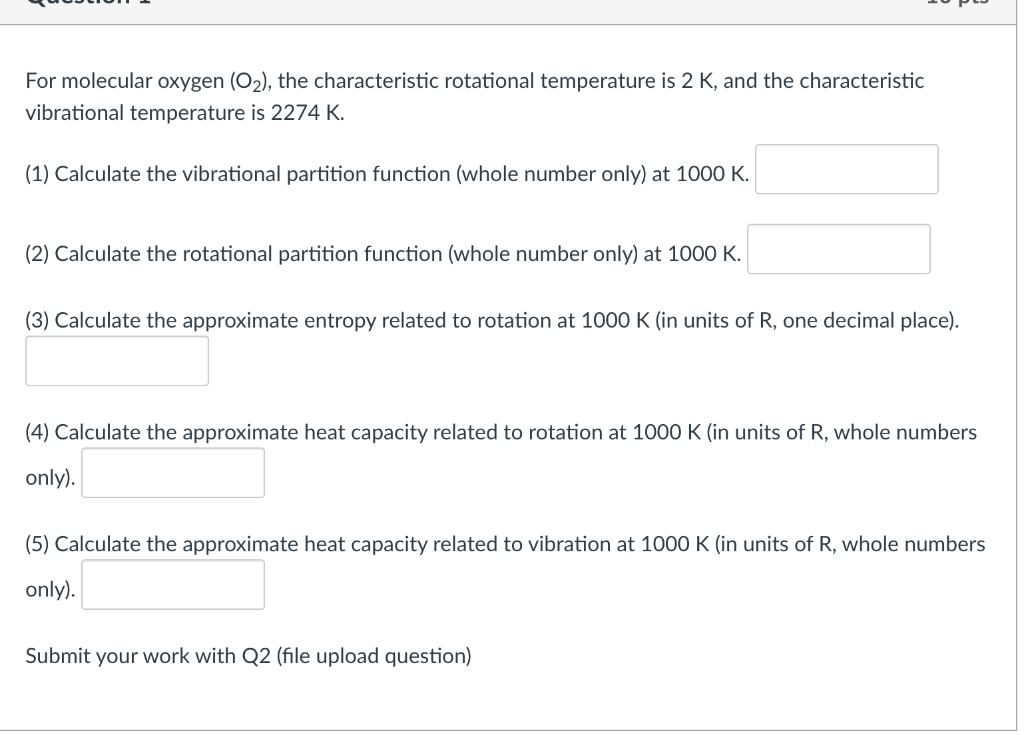
For molecular oxygen (O2), the characteristic rotational temperature is 2 K, and the characteristic vibrational temperature is 2274 K. (1) Calculate the vibrational partition function (whole number only) at 1000 K. (2) Calculate the rotational partition function (whole number only) at 1000 K. (3) Calculate the approximate entropy related to rotation at 1000 K (in units of R, one decimal place). (4) Calculate the approximate heat capacity related to rotation at 1000 K (in units of R, whole numbers only). (5) Calculate the approximate heat capacity related to vibration at 1000 K (in units of R, whole numbers only). Submit your work with Q2 (file upload question)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


