Question: PLEASE ANSWER ALL QUESTIONS ! Its 3 Different Questions . THANK YOU A mixture of xenon and neon gas is compressed from a volume of


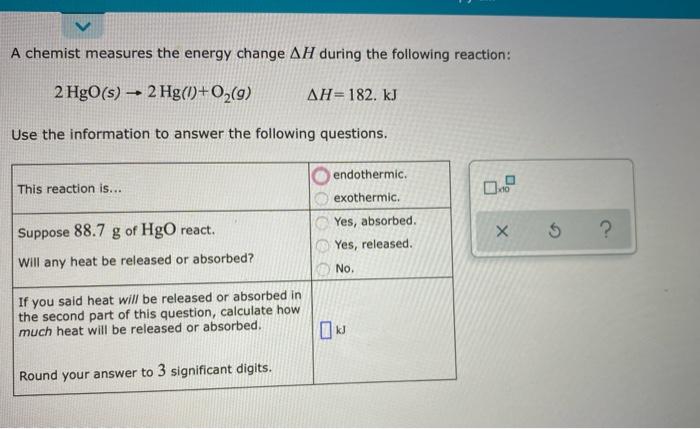
A mixture of xenon and neon gas is compressed from a volume of 92.0 L to a volume of 21.0 L, while the pressure is held constant at 72.0 atm. Calculate the work done on the gas mixture. Round your answer to 3 significant digits, and be sure it has the correct sign (positive or negative) 5 ? A chemist carefully measures the amount of heat needed to raise the temperature of a 0.10 kg sample of C,H,O, from 37.5 "C to 479 "C. The experiment shows that 1.77*10') of heat are needed. What can the chemist report for the molar heat capacity of CH4 0,7 Round your answer to 2 significant digits. 1 OP X 5 ? A chemist measures the energy change AH during the following reaction: 2 HgO(s) 2 Hg()+02(9) - AH=182. kJ Use the information to answer the following questions. endothermic. This reaction is... 0," exothermic. Yes, absorbed. Yes, released. X Suppose 88.7 g of HgO react. Will any heat be released or absorbed? $ No. If you said heat will be released or absorbed in the second part of this question, calculate how much heat will be released or absorbed. Round your answer to 3 significant digits
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


