Question: Please answer all questions, thank you!! TUTOR Predicting Relative lonization Energy IA BA H 2 3A4A5A6A7A He u Be BCN O F Ne Na Mg
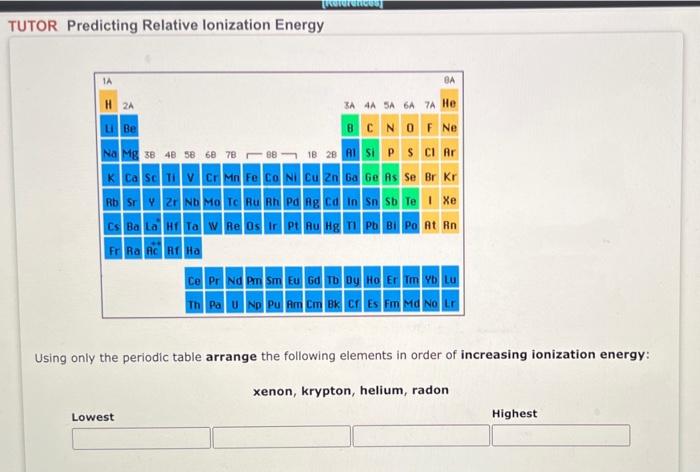
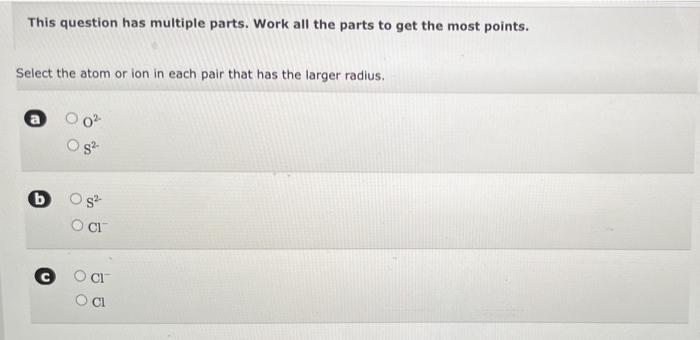
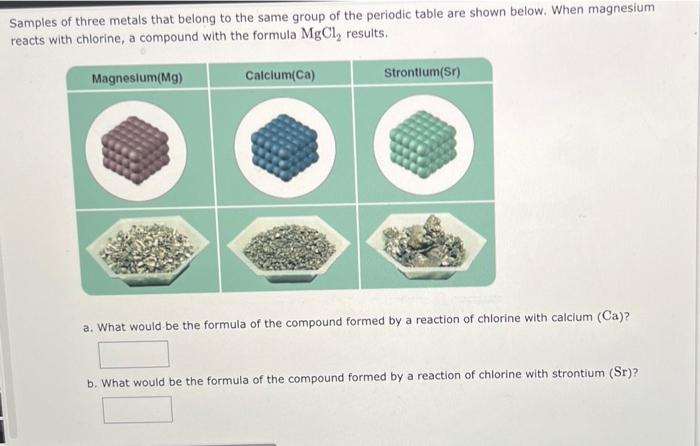
TUTOR Predicting Relative lonization Energy IA BA H 2 3A4A5A6A7A He u Be BCN O F Ne Na Mg 38 48 58 68 78 98 18 20 L S P S Cl Ar K Ca Sc V Cr Mn Fe Co N C 2n Ga Ge As Se Br Kr Rb SY 2ND MO TO RUR Pag Can Sb Te I Xe Cs Ba La Ta W Re Os La PL RU Hgn Pa Bi Po At Rn FRA G RHa Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb Lu Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No LE Using only the periodic table arrange the following elements in order of increasing ionization energy: xenon, krypton, helium, radon Lowest Highest This question has multiple parts. Work all the parts to get the most points. Select the atom or ion in each pair that has the larger radius. 82 b s? CI CI Samples of three metals that belong to the same group of the periodic table are shown below. When magnesium reacts with chlorine, a compound with the formula MgCl, results. Magnesium (Mg) Calcium(Ca) Strontium(sr) a. What would be the formula of the compound formed by a reaction of chlorine with calcium (Ca)? b. What would be the formula of the compound formed by a reaction of chlorine with strontium (Sr)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


