Question: please answer all questions. this is midterm practice that i do not understand please include all symmetry elements such as C2, E, sigma, etc and
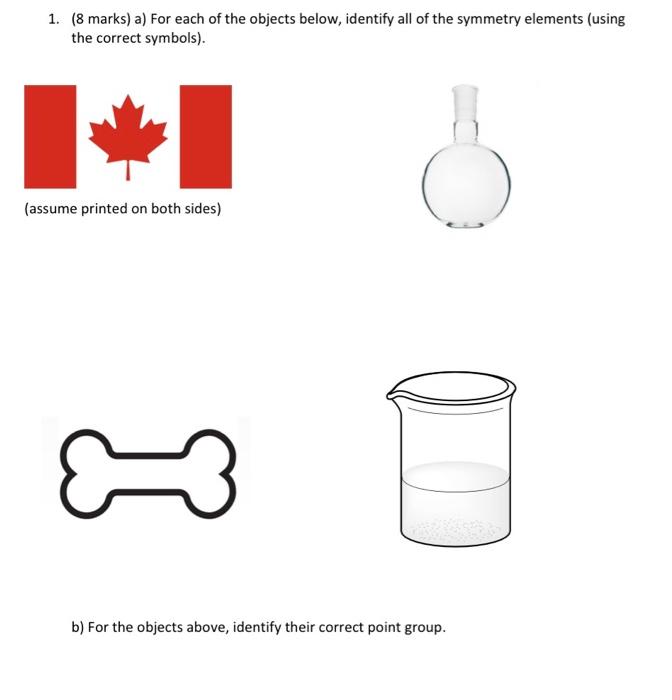

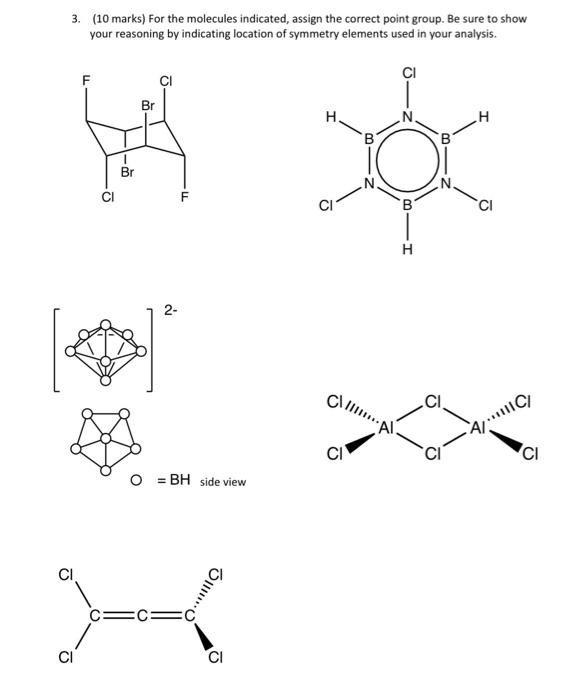

1. (8 marks) a) For each of the objects below, identify all of the symmetry elements (using the correct symbols). (assume printed on both sides) b) For the objects above, identify their correct point group. 2. (12 marks) For each of the molecules below, identify the correct VSEPR structure and identify all of the symmetry elements present (use the correct symbols) and identify the point group. a) CS2 b) NHBr2 c) Mezs* (consider Me groups as spheres) d) XeO2Cla (hypothetical) 3. (10 marks) For the molecules indicated, assign the correct point group. Be sure to show your reasoning by indicating location of symmetry elements used in your analysis. Br H. I B B z F CI B CI H 2- C.. A/IC/ CI CI = BH side view mo CI CI 4. (5 marks) a) White phosphorus is a tetrahedral molecule with the corresponding point group Td. It is an important industrial chemical for the production of many organo- phosporus molecules. It has been shown possible recently to replace one with an As to yield Asp, and AszP2 has also been proposed. Draw the correct structures for these and assign the proper point group. For the latter, be sure to indicate your reasoning. b) Consider the cyclo-triphosphinophosphonium ion the Lewis structure for which is illustrated. Draw the VSEPR structures for the two stereoisomers possible, identify the symmetry elements present and assign the correct point group (consider the methyl groups as spherical substituents). : PP
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


