Question: please answer all STP (standard temperature and pressure) is used as a reference point for the molar volumn of an ideal gas. In the USSA,

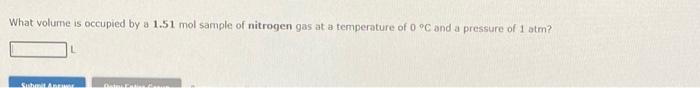

STP (standard temperature and pressure) is used as a reference point for the molar volumn of an ideal gas. In the USSA, mott chemicts, most general chemistry texts, and owi use STP=0C,1atm, where the molar volume =22.41/mol. If the reference preswire is chosen to be 1 bar, the inolar volume is 22.7L/mol. Do nat confuse the two. A 1.67 nwol sample of helium goss occupies a volume of l atsip. What volume is occupied by a 1.51mol sample of nitrogen gas at a temperature of 0C and a pressure of 1 atm? 1. Use the Aleferences to access important values if needed for this question. A sample of nitrogen gas that occupies a volume of 32.2 L at a temperature of 0 o Cand a pressure of 1 atm contains moles of gas
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


