Question: please answer all the question PROBLEM 4 The following is the equilibrium curve of CO2 absorption in 30% monoethanol amine (MEA) solution at room temperature
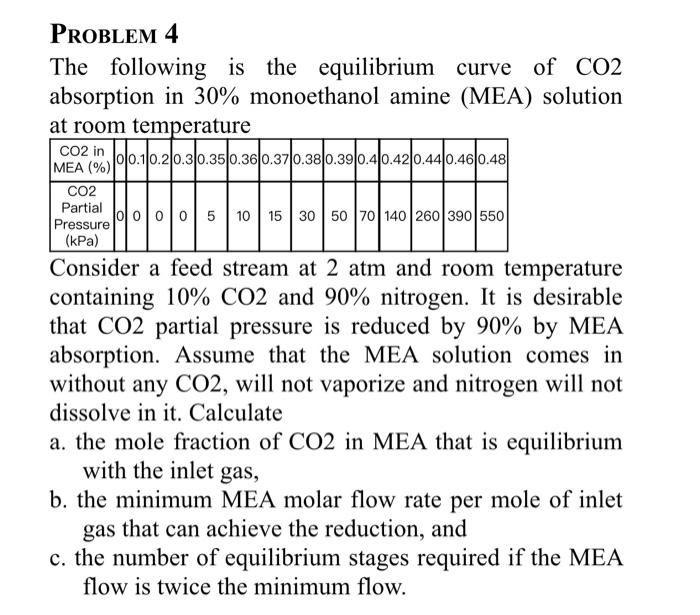
PROBLEM 4 The following is the equilibrium curve of CO2 absorption in 30% monoethanol amine (MEA) solution at room temperature Consider a feed stream at 2atm and room temperature containing 10%CO2 and 90% nitrogen. It is desirable that CO2 partial pressure is reduced by 90% by MEA absorption. Assume that the MEA solution comes in without any CO2, will not vaporize and nitrogen will not dissolve in it. Calculate a. the mole fraction of CO2 in MEA that is equilibrium with the inlet gas, b. the minimum MEA molar flow rate per mole of inlet gas that can achieve the reduction, and c. the number of equilibrium stages required if the MEA flow is twice the minimum flow
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


