Question: please answer all the questions Part B: Written Response Use the following information to answer the next question. lodine-131 is a radioactive element used in
please answer all the questions
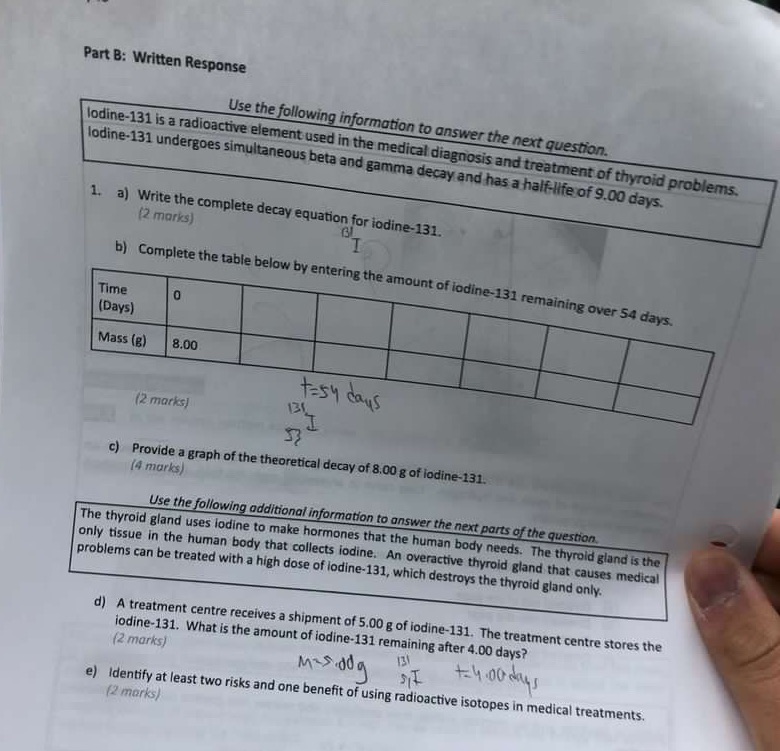
Part B: Written Response Use the following information to answer the next question. lodine-131 is a radioactive element used in the medical diagnosis and treatment of thyroid problems. lodine-131 undergoes simultaneous beta and gamma decay and has a half-life of 9.00 days. 1. a) Write the complete decay equation for iodine-131. (2 marks) BL b) Complete the table below by entering the amount of iodine-131 remaining over 54 days. Time 0 (Days) Mass (B) 8.00 t=sy cans (2 marks) 131 53 c) Provide a graph of the theoretical decay of 8.00 g of lodine-131. (4 marks) Use the following additional information to answer the next parts of the question. The thyroid gland uses lodine to make hormones that the human body needs. The thyroid gland is the only tissue in the human body that collects iodine. An overactive thyroid gland that causes medical problems can be treated with a high dose of lodine-131, which destroys the thyroid gland only. d) A treatment centre receives a shipment of 5.00 g of iodine-131. The treatment centre stores the iodine-131. What is the amount of iodine-131 remaining after 4.00 days? (2 marks) Mrs.dog 131 += 4:00 days e) Identify at least two risks and one benefit of using radioactive isotopes in medical treatments. (2 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


