Question: please answer all three questions clearly i will give rate. Which structure is the MRC and why? Be sure to select both of your answers.
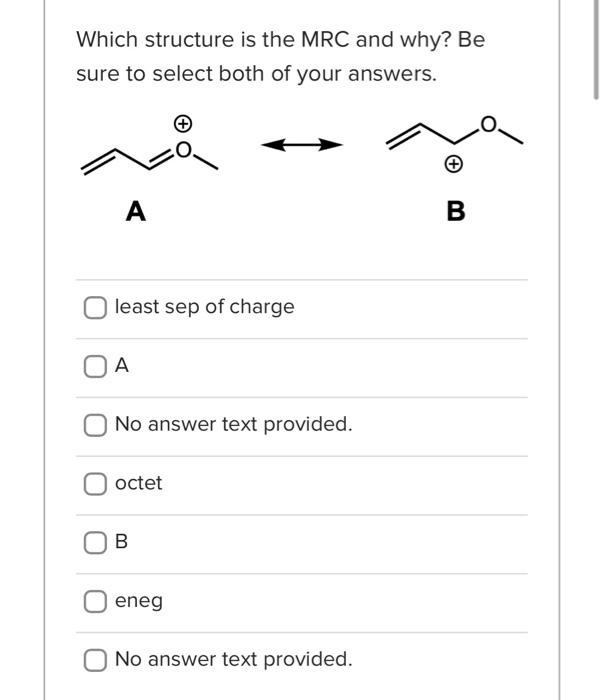
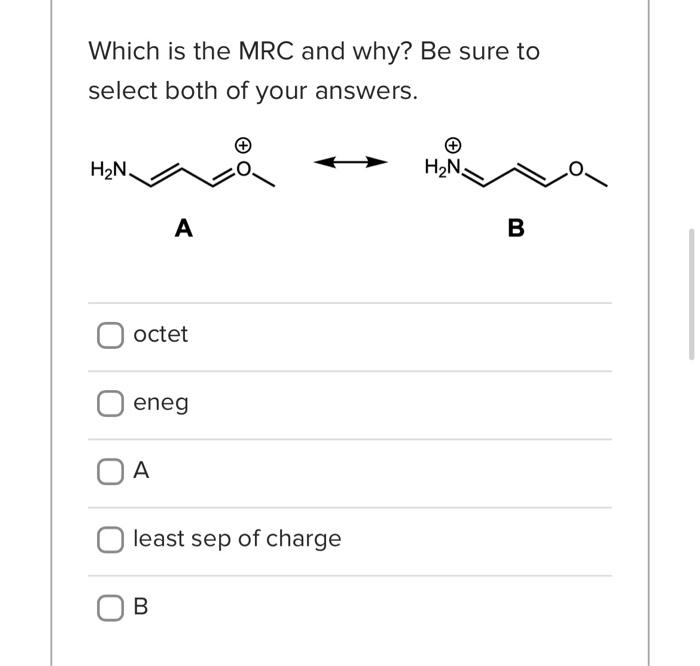
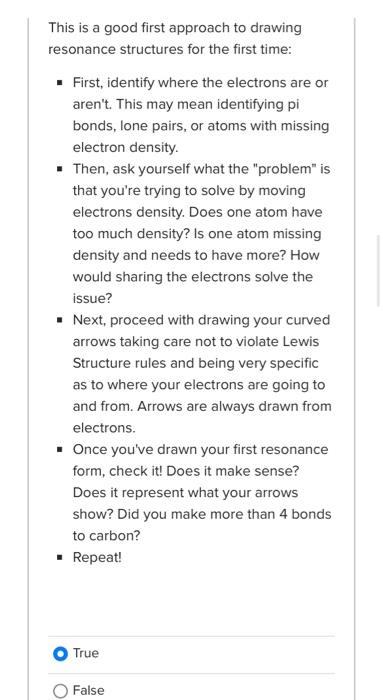
Which structure is the MRC and why? Be sure to select both of your answers. A B least sep of charge A No answer text provided. octet B eneg No answer text provided. Which is the MRC and why? Be sure to select both of your answers. A B octet eneg A least sep of charge This is a good first approach to drawing resonance structures for the first time: - First, identify where the electrons are or aren't. This may mean identifying pi bonds, lone pairs, or atoms with missing electron density. - Then, ask yourself what the "problem" is that you're trying to solve by moving electrons density. Does one atom have too much density? Is one atom missing density and needs to have more? How would sharing the electrons solve the issue? - Next, proceed with drawing your curved arrows taking care not to violate Lewis Structure rules and being very specific as to where your electrons are going to and from. Arrows are always drawn from electrons. - Once you've drawn your first resonance form, check it! Does it make sense? Does it represent what your arrows show? Did you make more than 4 bonds to carbon? - Repeat! True False
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


