Question: please answer all True or False questions False or True: 1. In the Molecular Orbital theory, electrons are treated as spreading throughout the entire molecule.
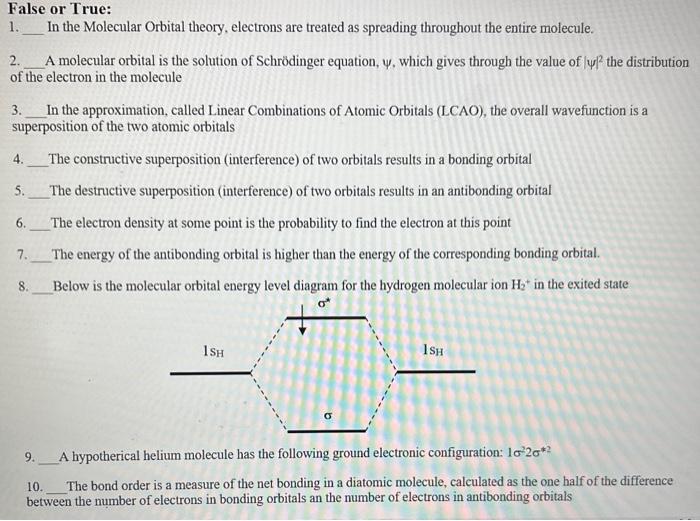
False or True: 1. In the Molecular Orbital theory, electrons are treated as spreading throughout the entire molecule. 2. A molecular orbital is the solution of Schrdinger equation, , which gives through the value of 2 the distribution of the electron in the molecule 3. In the approximation, called Linear Combinations of Atomic Orbitals (LCAO), the overall wavefunction is a superposition of the two atomic orbitals 4. The constructive superposition (interference) of two orbitals results in a bonding orbital 5. The destructive superposition (interference) of two orbitals results in an antibonding orbital 6. The electron density at some point is the probability to find the electron at this point 7. The energy of the antibonding orbital is higher than the energy of the corresponding bonding orbital. 8. Below is the molecular orbital energy level diagram for the hydrogen molecular ion H2+in the exited state 9. A hypotherical helium molecule has the following ground electronic configuration: 1222 10. The bond order is a measure of the net bonding in a diatomic molecule, calculated as the one half of the difference between the number of electrons in bonding orbitals an the number of electrons in antibonding orbitals
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


