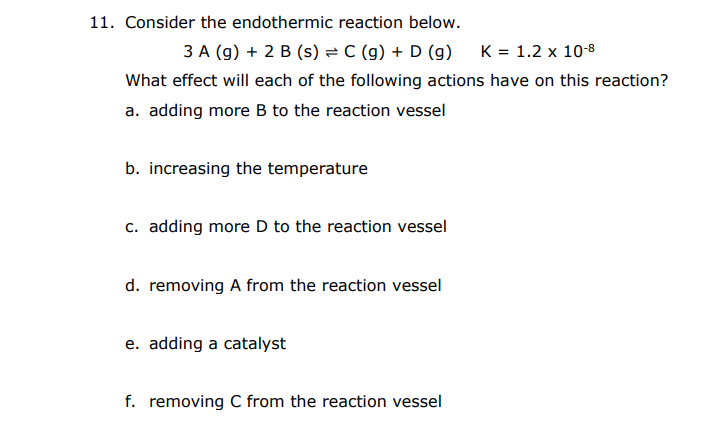
Question: please answer all, tysm. 11. Consider the endothermic reaction below. 3A(g)+2B(s)C(g)+D(g)K=1.2108 What effect will each of the following actions have on this reaction? a. adding
 please answer all, tysm.
please answer all, tysm.
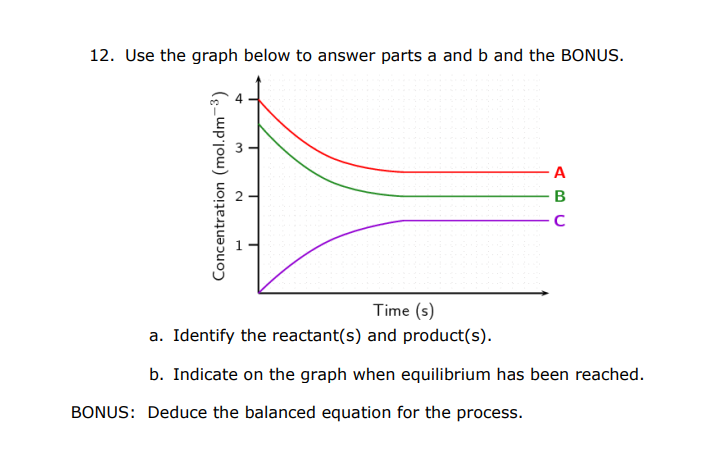
11. Consider the endothermic reaction below. 3A(g)+2B(s)C(g)+D(g)K=1.2108 What effect will each of the following actions have on this reaction? a. adding more B to the reaction vessel b. increasing the temperature c. adding more D to the reaction vessel d. removing A from the reaction vessel e. adding a catalyst f. removing C from the reaction vessel 12. Use the graph below to answer parts a and b and the BONUS. a. Identify the reactant(s) and product(s). b. Indicate on the graph when equilibrium has been reached. BONUS: Deduce the balanced equation for the process
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


