Question: please answer all:) will upvote TUTOR Converting Mass to Moles Using Molecular Weight II ? Tin (Sn) is element 50 on the periodic table. Calculate
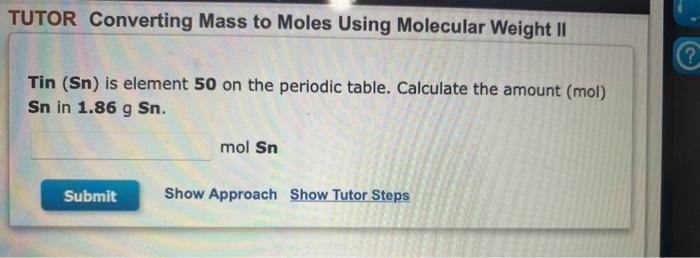
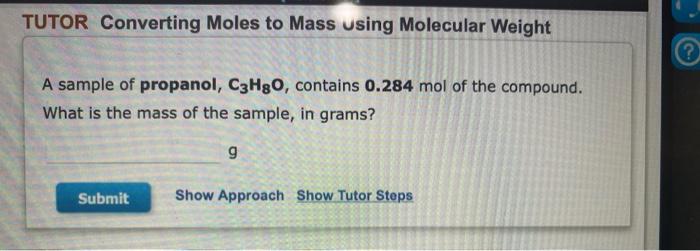
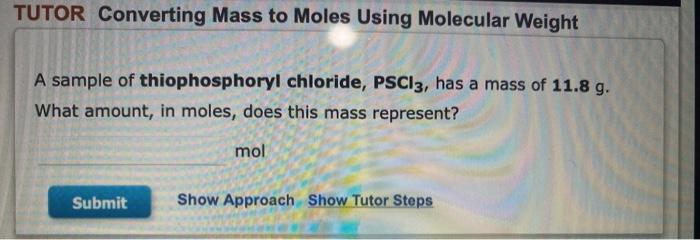

TUTOR Converting Mass to Moles Using Molecular Weight II ? Tin (Sn) is element 50 on the periodic table. Calculate the amount (mol) Sn in 1.86 g Sn. mol Sn Submit Show Approach Show Tutor Steps TUTOR Converting Moles to Mass using Molecular Weight A sample of propanol, C3Hgo, contains 0.284 mol of the compound. What is the mass of the sample, in grams? g Submit Show Approach Show Tutor Steps TUTOR Converting Mass to Moles Using Molecular Weight A sample of thiophosphoryl chloride, PSCI3, has a mass of 11.8 g. What amount, in moles, does this mass represent? mol Submit Show Approach Show Tutor Steps TUTOR Converting Number of Atoms to Moles Argon (Ar) is element 18 on the periodic table. A sample contains 3.50x1025 atoms of Ar. Calculate the amount of Ar. mol Ar
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


