Question: Calculate the oxidation state of the underlined element of the following compounds 1. CO2 2. SO, 16. RbO, 17. , 18. NH,OH 19. N,H,
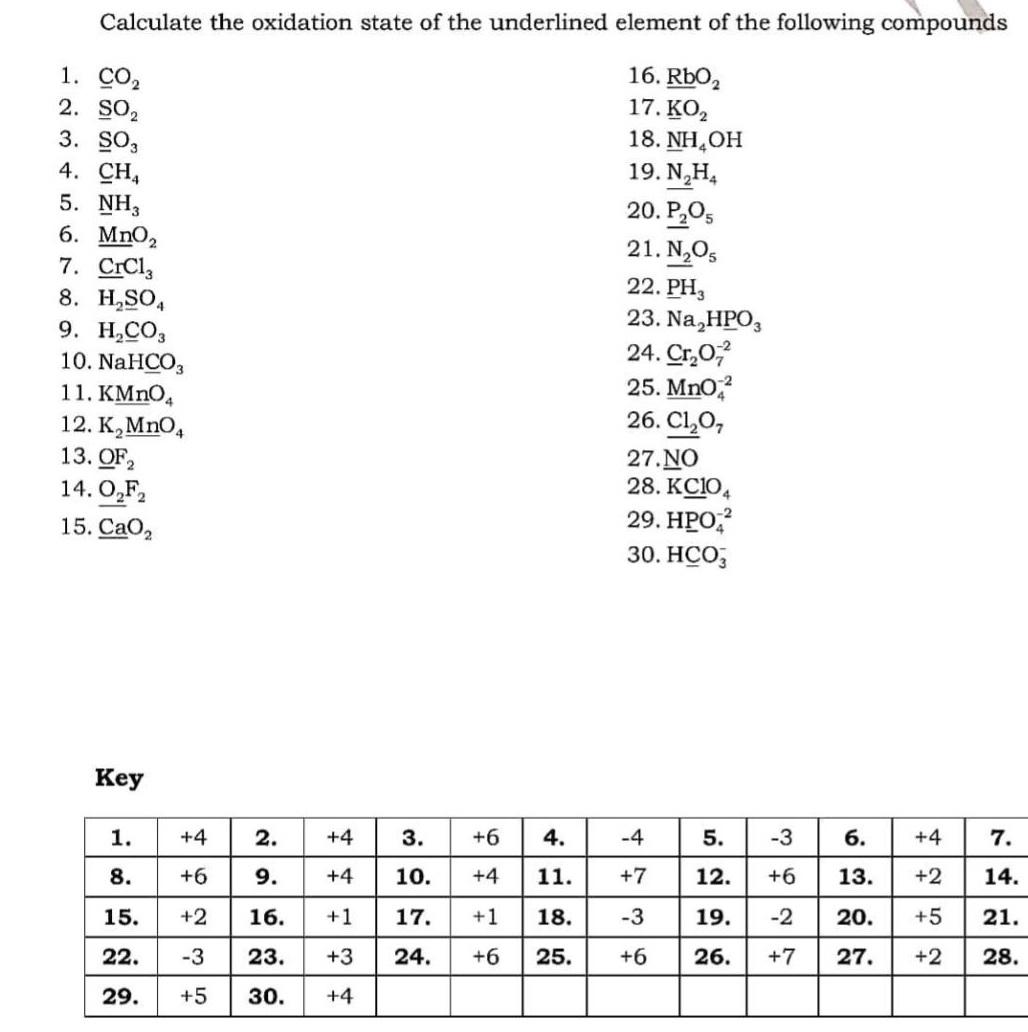
Calculate the oxidation state of the underlined element of the following compounds 1. CO2 2. SO, 16. RbO, 17. , 18. NH,OH 19. N,H, 3. SO, 4. CH, 5. NH, 6. MnO, 7. CrCl, 8. H,SO, 9. ., 10. NaHCO3 20. ,, 21. N,O, 22. PH, 23. Na,HPO, 24. Cr,0, 25. Mno, 26. Cl,0, 11. KMNO, 12. , MnO, 13. OF2 14. O,F, 15. CaO, 27. NO 28. KC1O, 29. , 30. , ey 1. +4 2. +4 3. +6 4. -4 5. -3 . +4 7. 8. +6 9. +4 10. +4 11. +7 12. +6 13. +2 14. 15. +2 16. +1 17. +1 18. -3 19. -2 20. +5 21. 22. -3 23. +3 24. +6 25. +6 26. +7 27. +2 28. 29. +5 30. +4
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


