Question: please answer and explain question 1 Using the data in Table 1 calculate the Kp value for protonated benzoic acid. (note: Kp values have no
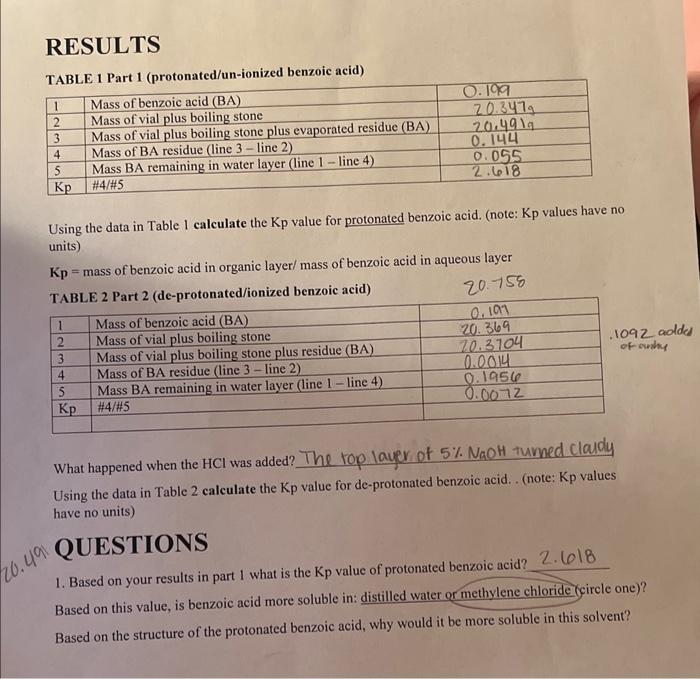
Using the data in Table 1 calculate the Kp value for protonated benzoic acid. (note: Kp values have no units) Kp = mass of benzoic acid in organic layer/ mass of benzoic acid in aqueous layer What happened when the HCl was added? The top layer of 5%NaOH turned claldy Using the data in Table 2 calculate the Kp value for de-protonated benzoic acid. . (note: Kp values have no units) QUESTIONS 1. Based on your results in part 1 what is the Kp value of protonated benzoic acid? 2.618 Based on this value, is benzoic acid more soluble in: distilled water or methylene chloride (circle one)? Based on the structure of the protonated benzoic acid, why would it be more soluble in this solvent
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


