Question: Please answer and show all work so I can understand how to work this problem. Compare the Balmer series of hydrogen with the series where
Please answer and show all work so I can understand how to work this problem.
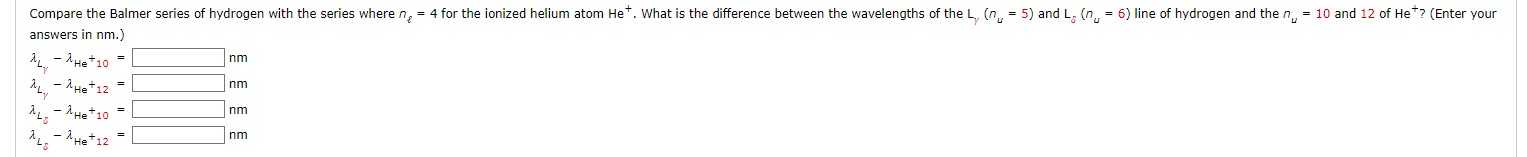
Compare the Balmer series of hydrogen with the series where ne = 4 for the ionized helium atom He. What is the difference between the wavelengths of the L (n,, = 5) and Ly (n,, = 6) line of hydrogen and the n, = 10 and 12 of He*? (Enter your answers in nm.) - XHe + 10 nm - AHe+ 12 nm My - He + 10 = nm nm - AHe+ 12 =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


