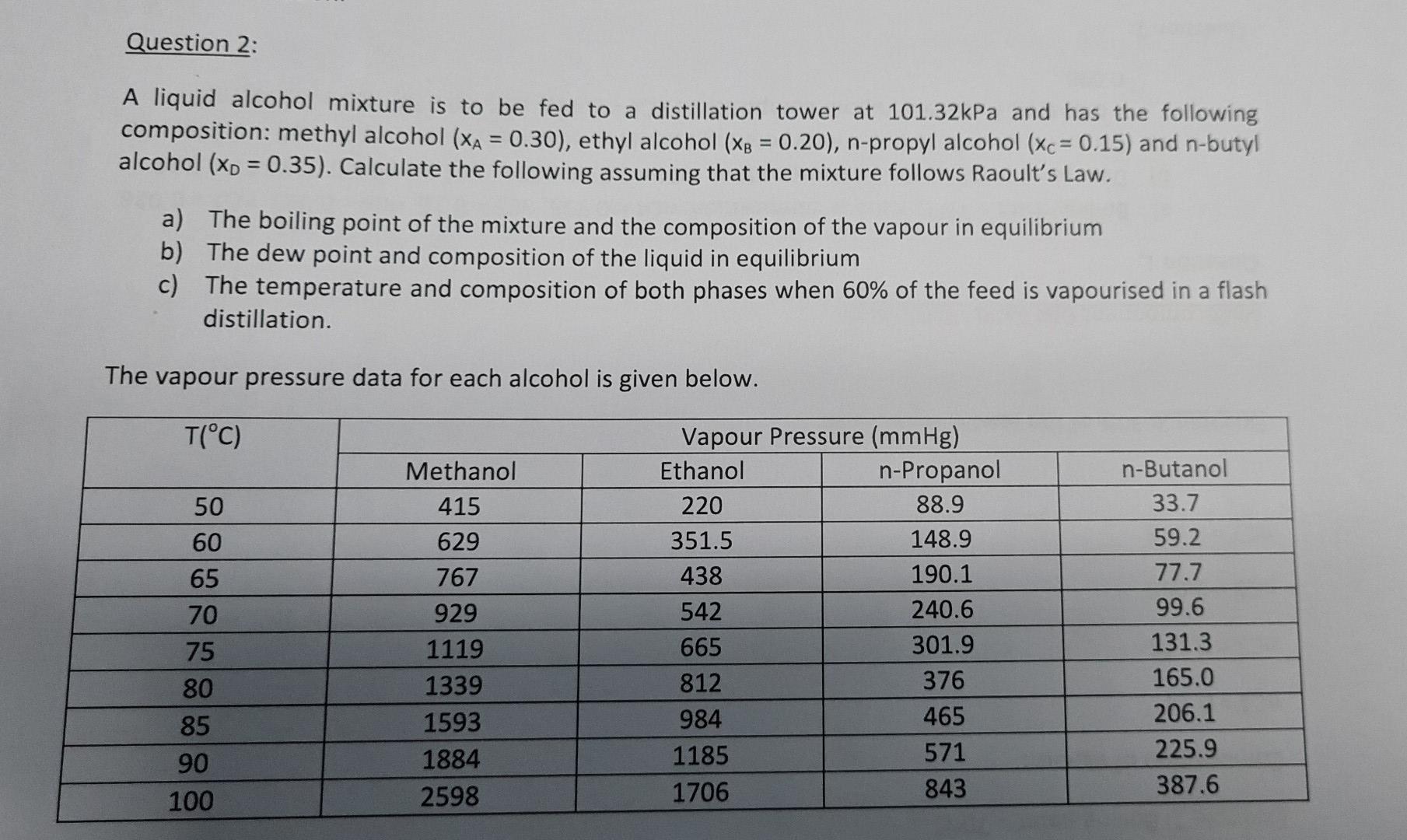
Question: please answer as detailed as possible Question 2: A liquid alcohol mixture is to be fed to a distillation tower at 101.32kPa and has the
please answer as detailed as possible
Question 2: A liquid alcohol mixture is to be fed to a distillation tower at 101.32kPa and has the following composition: methyl alcohol (XA = 0.30), ethyl alcohol (xp = 0.20), n-propyl alcohol (Xc = 0.15) and n-butyl alcohol (xp = 0.35). Calculate the following assuming that the mixture follows Raoult's Law. - a) The boiling point of the mixture and the composition of the vapour in equilibrium b) The dew point and composition of the liquid in equilibrium c) The temperature and composition of both phases when 60% of the feed is vapourised in a flash distillation. The vapour pressure data for each alcohol is given below. T(C) 50 60 65 70 75 80 85 90 100 Methanol 415 629 767 929 1119 1339 1593 1884 2598 Vapour Pressure (mmHg) Ethanol n-Propanol 220 88.9 351.5 148.9 438 190.1 542 240.6 665 301.9 812 376 984 465 1185 571 1706 843 n-Butanol 33.7 59.2 77.7 99.6 131.3 165.0 206.1 225.9 387.6
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


