Question: please answer both and include detailed steps 4) How many moles of HIO3 can be produced from the reaction of 635g of iodine trichloride and
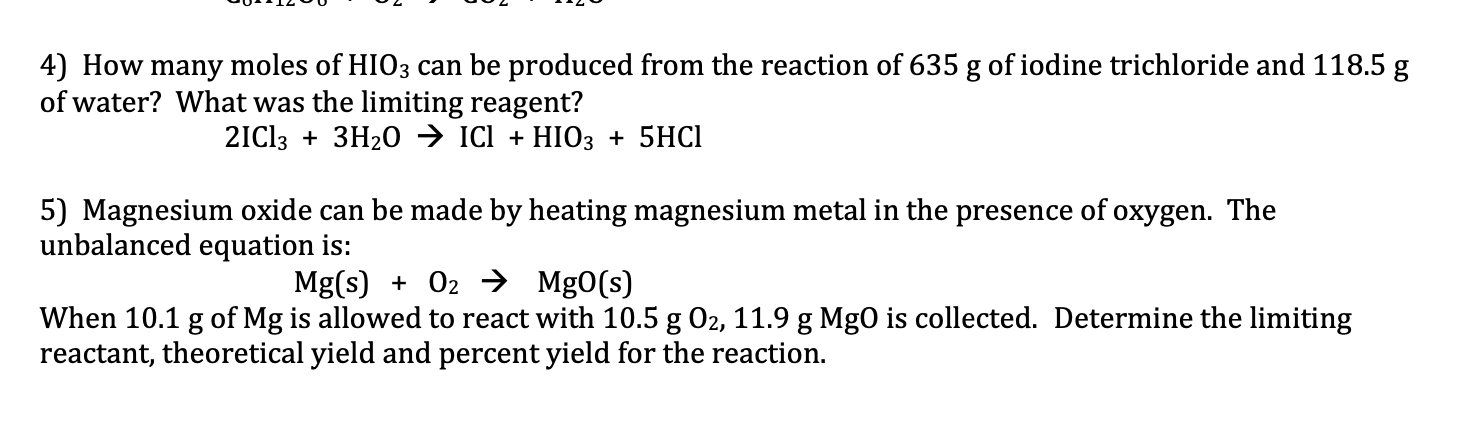 please answer both and include detailed steps
please answer both and include detailed steps
4) How many moles of HIO3 can be produced from the reaction of 635g of iodine trichloride and 118.5g of water? What was the limiting reagent? 2ICl3+3H2OICl+HIO3+5HCl 5) Magnesium oxide can be made by heating magnesium metal in the presence of oxygen. The unbalanced equation is: Mg(s)+O2MgO(s) When 10.1g of Mg is allowed to react with 10.5gO2,11.9gMg0 is collected. Determine the limiting reactant, theoretical yield and percent yield for the reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


