Question: please answer both Determine the freezing point of the solution. (Use Kf=1.86C/m.) Express your answer in degrees Celsius to three significant figures. MISSED THIS? Read
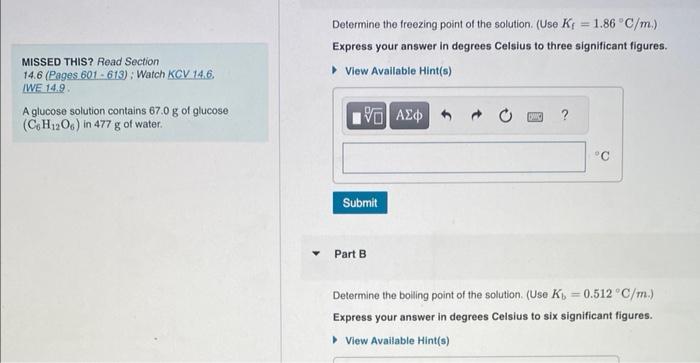
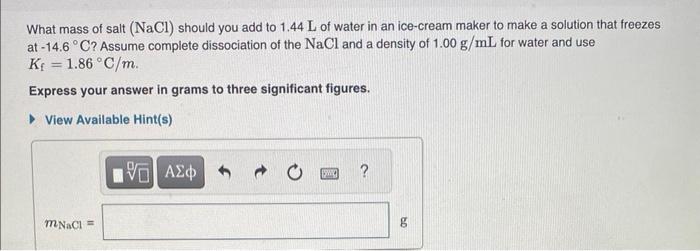
Determine the freezing point of the solution. (Use Kf=1.86C/m.) Express your answer in degrees Celsius to three significant figures. MISSED THIS? Read Section 14.6 (Pages 601 - 613) ; Watch KCV 14.6. IWE 14.9. A glucose solution contains 67.0g of glucose (C6H12O6) in 477g of water. Part B Determine the boiling point of the solution. (Use Kb=0.512C/m.) Express your answer in degrees Celsius to six significant figures. What mass of salt (NaCl) should you add to 1.44L of water in an ice-cream maker to make a solution that freezes at 14.6C ? Assume complete dissociation of the NaCl and a density of 1.00g/mL for water and use Kf=1.86C/m Express your answer in grams to three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


