Question: Please answer both! I need help Consider the following reaction where Kc=6.30 at 723K. 2NH3(g)N2(g)+3H2(g) A reaction mixture was found to contain 0.000665 moles of

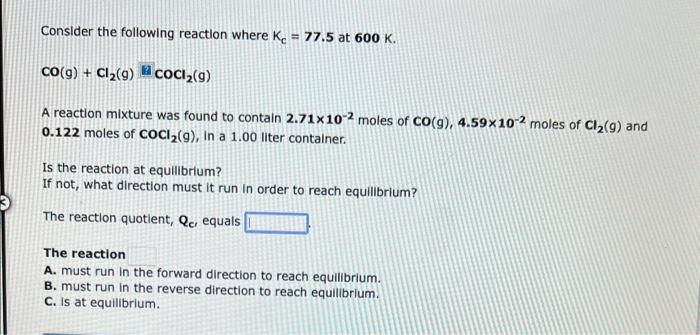
Consider the following reaction where Kc=6.30 at 723K. 2NH3(g)N2(g)+3H2(g) A reaction mixture was found to contain 0.000665 moles of NH3(g),0.0265 moles of N2(g), and 0.0422 moles of H2(g), in a 1.00 liter container. Calculate Qc. Qc= Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction must run in the forward direction to reach equilibrium. The reaction must run in the reverse direction to reach equilibrium. The reaction is at equilibrium. Consider the following reaction where Kc=77.5 at 600K. CO(g)+Cl2(g)=COCl2(g) A reaction mixture was found to contain 2.71102 moles of CO(g),4.59102 moles of Cl2(g) and 0.122 moles of COCl2(g), in a 1.00 liter container. Is the reaction at equilibrium? If not, what direction must it run in order to reach equilibrium? The reaction quotient, Qc, equals The reaction A. must run in the forward direction to reach equilibrium. B. must run in the reverse direction to reach equillbrium. C. is at equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


