Question: please answer both. I will give thumbs up Draw orbital diagrams for the following elements: (Not all blocks will be used) [5 Marks) Phosphorus: Is
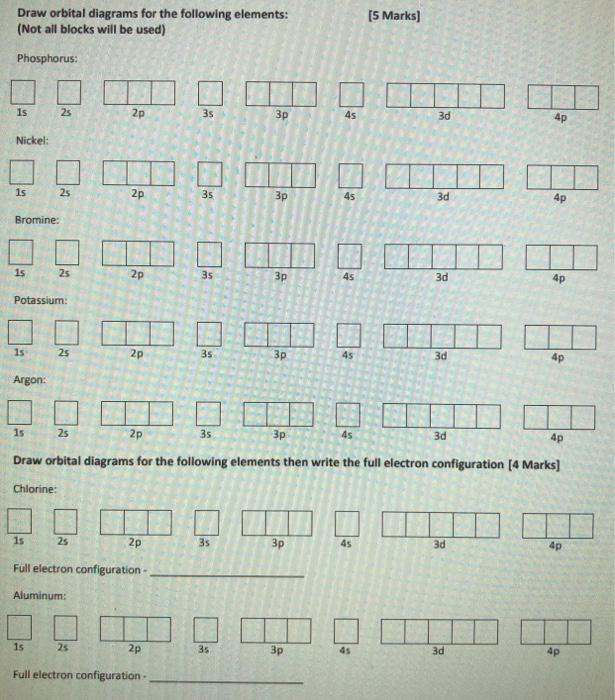
Draw orbital diagrams for the following elements: (Not all blocks will be used) [5 Marks) Phosphorus: Is 28 2p 35 3p 45 3d 4p Nickel: 1s 25 2p 35 45 3d 4p Bromine: 1s 25 2p 35 3p 45 3d 4p Potassium: 1s 25 2p 35 3p 45 3d 4p Argon: 1s 25 2p 3s 45 3d 4p Draw orbital diagrams for the following elements then write the full electron configuration [4 Marks] Chlorine: 15 2s 2p 3s 3p 45 3d 4p Full electron configuration - Aluminum: 1s 25 2p 35 3d 4p Full electron configuration
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


