Question: Please answer both separately A scientist measures the standard enthalpy change for the following reaction to be 736.2kJ : 2CO(g)+2NO(g)2CO2(g)+N2(g) Based on this value and
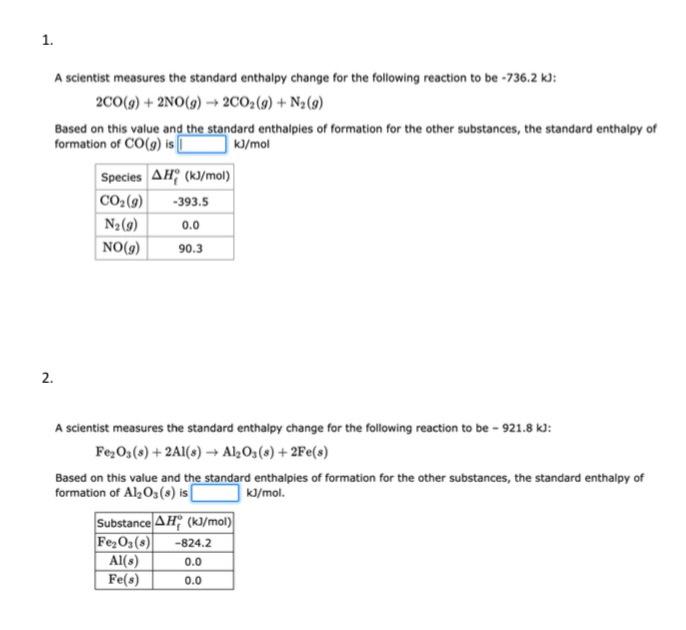
A scientist measures the standard enthalpy change for the following reaction to be 736.2kJ : 2CO(g)+2NO(g)2CO2(g)+N2(g) Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of CO(g) is kJ/mol 2. A scientist measures the standard enthalpy change for the following reaction to be 921.8kJ : Fe2O3(s)+2Al(s)Al2O3(s)+2Fe(s) Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of Al2O3(s) is kJ/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


