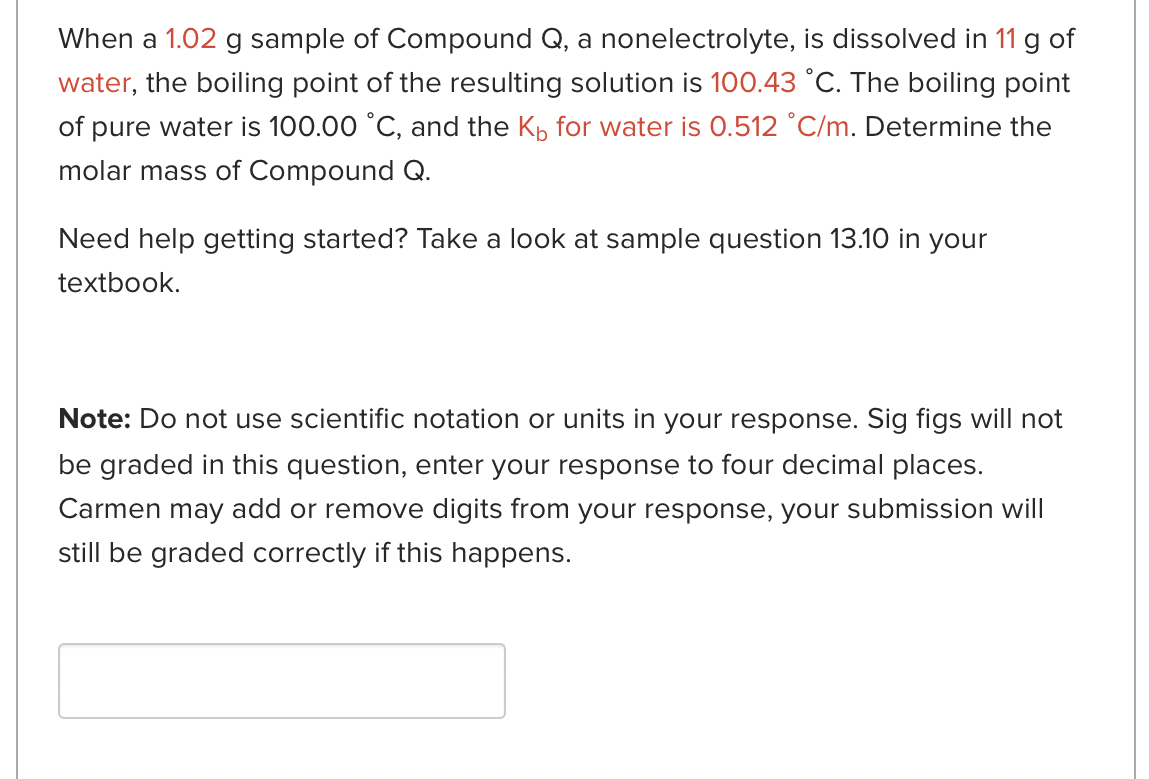
Question: please answer both, thank you!! When a 1.02g sample of Compound Q, a nonelectrolyte, is dissolved in 11g of water, the boiling point of the
 please answer both, thank you!!
please answer both, thank you!!
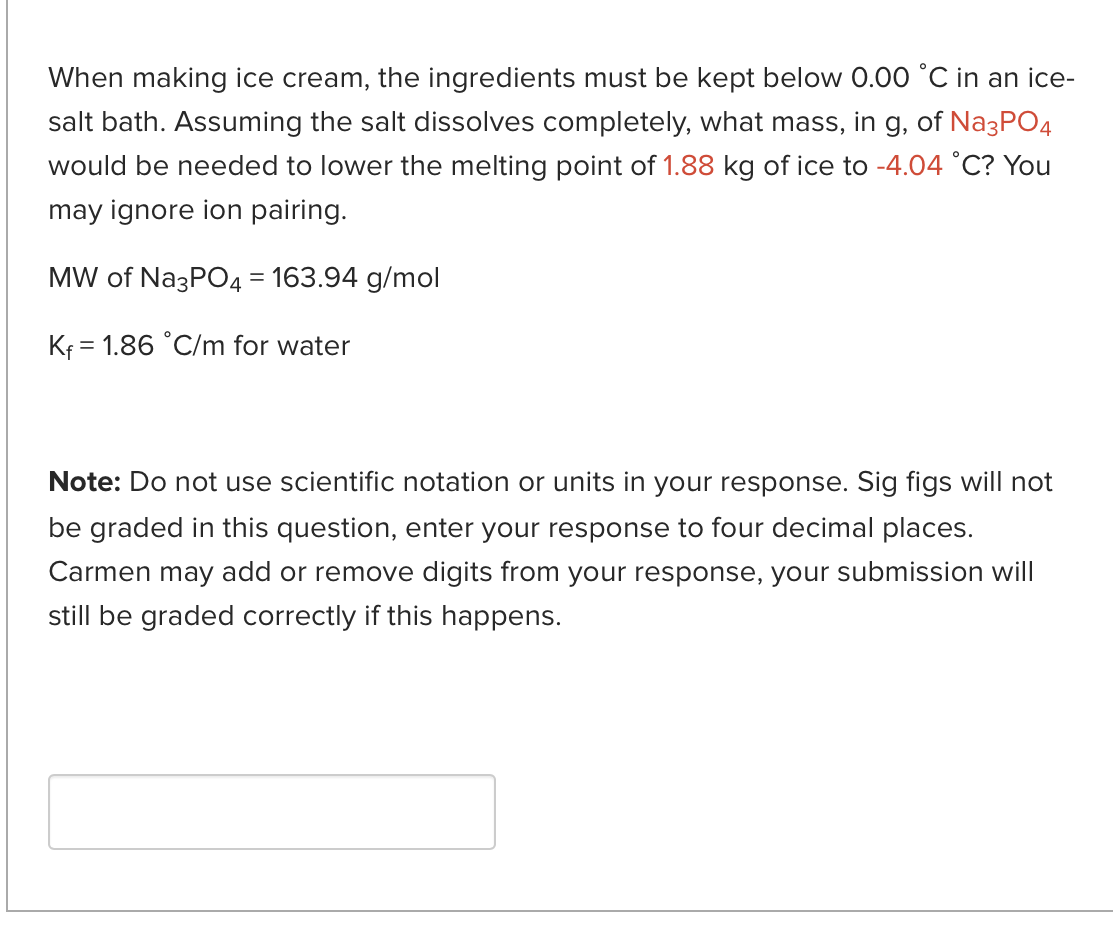
When a 1.02g sample of Compound Q, a nonelectrolyte, is dissolved in 11g of water, the boiling point of the resulting solution is 100.43C. The boiling point of pure water is 100.00C, and the Kb for water is 0.512C/m. Determine the molar mass of Compound Q. Need help getting started? Take a look at sample question 13.10 in your textbook. Note: Do not use scientific notation or units in your response. Sig figs will not be graded in this question, enter your response to four decimal places. Carmen may add or remove digits from your response, your submission will still be graded correctly if this happens. When making ice cream, the ingredients must be kept below 0.00C in an icesalt bath. Assuming the salt dissolves completely, what mass, in g, of Na3PO4 would be needed to lower the melting point of 1.88kg of ice to 4.04C ? You may ignore ion pairing. MW of Na3PO4=163.94g/mol Kf=1.86C/m for water Note: Do not use scientific notation or units in your response. Sig figs will not be graded in this question, enter your response to four decimal places. Carmen may add or remove digits from your response, your submission will still be graded correctly if this happens
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


