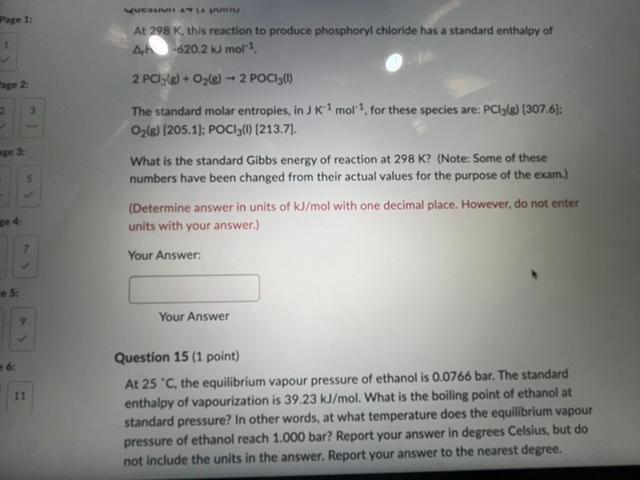
Question: please answer both, will give a thumbs up At 298K, this reaction to produce phosphoryl chloride has a standard enthalpy of A. h 620.2kJmol1 2PCl3(s)+O2(g)2POCl3(1)
 please answer both, will give a thumbs up
please answer both, will give a thumbs up
At 298K, this reaction to produce phosphoryl chloride has a standard enthalpy of A. h 620.2kJmol1 2PCl3(s)+O2(g)2POCl3(1) The standard molar entropies, in JK1mol1, for these species are: PCl3(e)(307.6) : O2(g)[205.1];POCl3(1)[213.7]. What is the standard Gibbs energy of reaction at 298K ? (Note: Some of these numbers have been changed from their actual values for the purpose of the exam.) (Determine answer in units of kJ/mol with one decimal place. However, do not enter units with your answer.) Your Answer: Your Answer Question 15 (1 point) At 25C, the equilibrium vapour pressure of ethanol is 0.0766 bar. The standard enthalpy of vapourization is 39.23kJ/mol. What is the boiling point of ethanol at standard pressure? In other words, at what temperature does the equilibrium vapour pressure of ethanol reach 1.000 bar? Report your answer in degrees Celsius, but do not include the units in the answer. Report your answer to the nearest degree
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


