Question: PLEASE ANSWER BOTH WILL RATE HIGHLY!! Q2) Consider the following equation 2A(g) + B(g) 3C(g), A flask at 298 K initially contains 0.185 M of
PLEASE ANSWER BOTH WILL RATE HIGHLY!!
Q2)
Consider the following equation
2A(g) + B(g) 3C(g),
A flask at 298 K initially contains 0.185 M of A and 0.233 M of B. At equilibrium the flask contains 0.215 M of C. Determine the value of the equilibrium constant for this reaction at 298K.
Group of answer choices
613
79.9
5.72
1.25
35.5
- 17 x 10-2
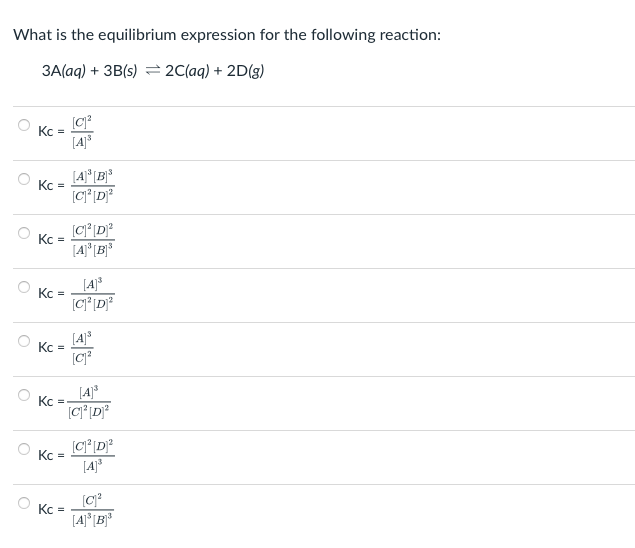
What is the equilibrium expression for the following reaction: 3A(aq) + 3B(s) = 2 (aq) + 2D(g) Kc = [4] Kc = [4] [B] C'D Kc = C'D [A] [B [4] Kc = [C'D Kc = [A] [C? O [4] Kc = [ CD] Kc = C'D [4] Kc ( [A] [B]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


