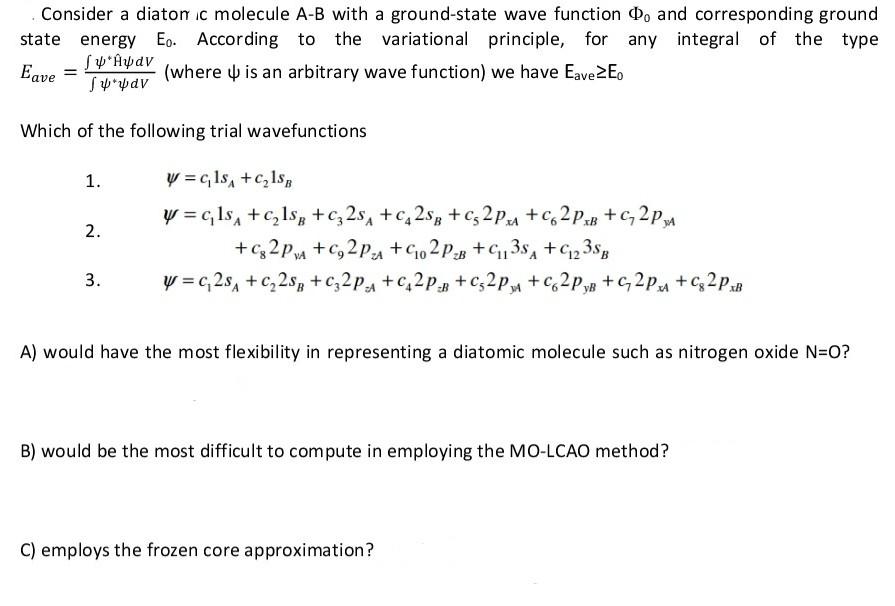
Question: Please answer Consider a diatonic molecule A-B with a ground-state wave function Do and corresponding ground state energy Eo. According to the variational principle, for
Please answer
Consider a diatonic molecule A-B with a ground-state wave function Do and corresponding ground state energy Eo. According to the variational principle, for any integral of the type Eave [ Sp* pav (where is an arbitrary wave function) we have Eave Eo Which of the following trial wavefunctions 1. y=c1s +C1SB y=c1s +1$B + C 25 +C2SB + C2PA +C62PB+C2PA 2. +C82PMA +C2PA +C02PB +C13SA +C23SB 3. y=c2s +C2SB + C2PA +C2PB + C2PM +C62PB + C2PA +Cg2PxB A) would have the most flexibility in representing a diatomic molecule such as nitrogen oxide N=O? B) would be the most difficult to compute in employing the MO-LCAO method? C) employs the frozen core approximation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


