Question: please answer correct You and yeur lab parther are studying the rate of a reactien, A+B C. You make meaturements of the initial rate under
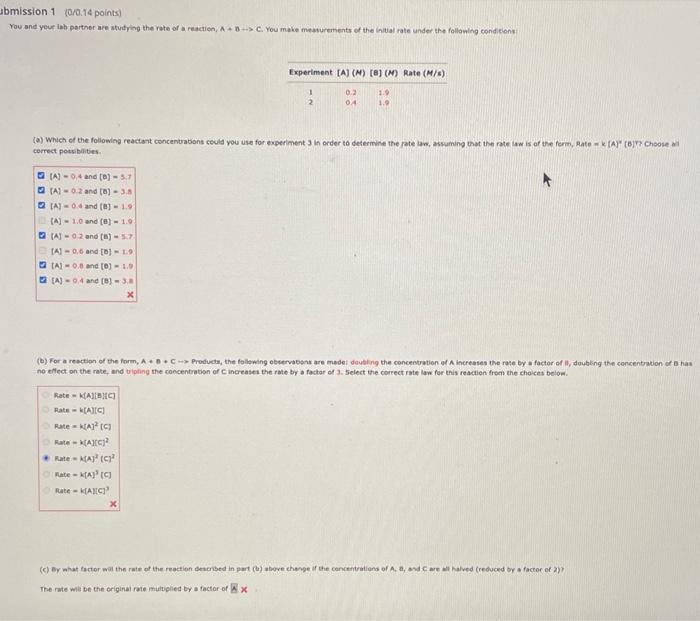
You and yeur lab parther are studying the rate of a reactien, A+B C. You make meaturements of the initial rate under the following condicens: cerrect pots balties. no effect on the rate, and biphing the concentration of C increases the rate by a factar of 3 . 5elect the coirect rate law for this reactien from the choices beiof. \begin{tabular}{l} Rate =k[A][a]}C] \\ Rate =k[A][C] \\ Rate =k[A]2[C] \\ Rate = N [A][C]2 \\ Rate =k(A]2[C]2 \\ Rate =k[A]3[C] \\ Rate =k[A][C]3 \\ \hline X \end{tabular} (c) By ahat factor wal the rate of the reaction described in part (b ) atore cherge if the cencentratiens of A, an, and C are wll halved (reduced by a factor of 2 ) ? The rate wie be the original rate multigled by a factor of xx
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


